TNK2/ACK1-mediated phosphorylation of ATP5F1A (ATP synthase F1 subunit alpha) selectively augments survival of prostate cancer while engendering mitochondrial vulnerability
- PMID: 35895804
- PMCID: PMC9980697
- DOI: 10.1080/15548627.2022.2103961
TNK2/ACK1-mediated phosphorylation of ATP5F1A (ATP synthase F1 subunit alpha) selectively augments survival of prostate cancer while engendering mitochondrial vulnerability
Abstract
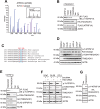
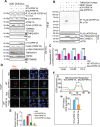
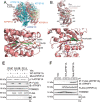
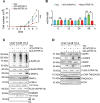
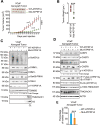
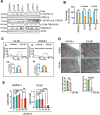
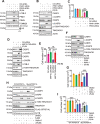
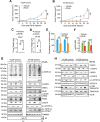
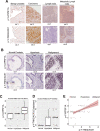
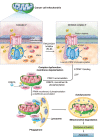
The challenge of rapid macromolecular synthesis enforces the energy-hungry cancer cell mitochondria to switch their metabolic phenotypes, accomplished by activation of oncogenic tyrosine kinases. Precisely how kinase activity is directly exploited by cancer cell mitochondria to meet high-energy demand, remains to be deciphered. Here we show that a non-receptor tyrosine kinase, TNK2/ACK1 (tyrosine kinase non receptor 2), phosphorylated ATP5F1A (ATP synthase F1 subunit alpha) at Tyr243 and Tyr246 (Tyr200 and 203 in the mature protein, respectively) that not only increased the stability of complex V, but also increased mitochondrial energy output in cancer cells. Further, phospho-ATP5F1A (p-Y-ATP5F1A) prevented its binding to its physiological inhibitor, ATP5IF1 (ATP synthase inhibitory factor subunit 1), causing sustained mitochondrial activity to promote cancer cell growth. TNK2 inhibitor, (R)-9b reversed this process and induced mitophagy-based autophagy to mitigate prostate tumor growth while sparing normal prostate cells. Further, depletion of p-Y-ATP5F1A was needed for (R)-9b-mediated mitophagic response and tumor growth. Moreover, Tnk2 transgenic mice displayed increased p-Y-ATP5F1A and loss of mitophagy and exhibited formation of prostatic intraepithelial neoplasia (PINs). Consistent with these data, a marked increase in p-Y-ATP5F1A was seen as prostate cancer progressed to the malignant stage. Overall, this study uncovered the molecular intricacy of tyrosine kinase-mediated mitochondrial energy regulation as a distinct cancer cell mitochondrial vulnerability and provided evidence that TNK2 inhibitors can act as "mitocans" to induce cancer-specific mitophagy.Abbreviations: ATP5F1A: ATP synthase F1 subunit alpha; ATP5IF1: ATP synthase inhibitory factor subunit 1; CRPC: castration-resistant prostate cancer; DNM1L: dynamin 1 like; MAP1LC3B/LC3B: microtubule associated protein 1 light chain 3 beta; Mdivi-1: mitochondrial division inhibitor 1; Mut-ATP5F1A: Y243,246A mutant of ATP5F1A; OXPHOS: oxidative phosphorylation; PC: prostate cancer; PINK1: PTEN induced kinase 1; p-Y-ATP5F1A: phosphorylated tyrosine 243 and 246 on ATP5F1A; TNK2/ACK1: tyrosine kinase non receptor 2; Ub: ubiquitin; WT: wild type.
Keywords: ATP5F1A; ATP5IF1; TNK2/ACK1; mitochondrial dysfunction; mitochondrial vulnerability; mitophagy; tyrosine phosphorylation.
Conflict of interest statement
Patents “Inhibitors of ACK1/TNK2 Tyrosine Kinase” (patent no. 9,850,216 and 10,017,478) cover (
Figures


Similar articles
-
Chimeric SFT2D2-TBX19 Promotes Prostate Cancer Progression by Encoding TBX19-202 Protein and Stabilizing Mitochondrial ATP Synthase through ATP5F1A Phosphorylation.Adv Sci (Weinh). 2024 Dec;11(48):e2408426. doi: 10.1002/advs.202408426. Epub 2024 Nov 14. Adv Sci (Weinh). 2024. PMID: 39540264 Free PMC article.
-
Aberrant mitochondrial morphology and function associated with impaired mitophagy and DNM1L-MAPK/ERK signaling are found in aged mutant Parkinsonian LRRK2R1441G mice.Autophagy. 2021 Oct;17(10):3196-3220. doi: 10.1080/15548627.2020.1850008. Epub 2020 Dec 10. Autophagy. 2021. PMID: 33300446 Free PMC article.
-
Alternative mitochondrial quality control mediated by extracellular release.Autophagy. 2021 Oct;17(10):2962-2974. doi: 10.1080/15548627.2020.1848130. Epub 2020 Dec 10. Autophagy. 2021. PMID: 33218272 Free PMC article.
-
ACK1/TNK2 tyrosine kinase: molecular signaling and evolving role in cancers.Oncogene. 2015 Aug 6;34(32):4162-7. doi: 10.1038/onc.2014.350. Epub 2014 Oct 27. Oncogene. 2015. PMID: 25347744 Free PMC article. Review.
-
Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles.Autophagy. 2021 Feb;17(2):385-401. doi: 10.1080/15548627.2020.1725377. Epub 2020 Feb 12. Autophagy. 2021. PMID: 32048886 Free PMC article. Review.
Cited by
-
TEP SNORD12B, SNORA63, and SNORD14E as novel biomarkers for hepatitis B virus-related hepatocellular carcinoma (HBV-related HCC).Cancer Cell Int. 2024 Jan 2;24(1):3. doi: 10.1186/s12935-023-03179-z. Cancer Cell Int. 2024. PMID: 38167096 Free PMC article.
-
Prostate cancer immunotherapy: Improving clinical outcomes with a multi-pronged approach.Cell Rep Med. 2023 Oct 17;4(10):101199. doi: 10.1016/j.xcrm.2023.101199. Epub 2023 Sep 21. Cell Rep Med. 2023. PMID: 37738978 Free PMC article. Review.
-
Suppression of Spry1 reduces HIF1α-dependent glycolysis and impairs angiogenesis in BRAF-mutant cutaneous melanoma.J Exp Clin Cancer Res. 2025 Feb 14;44(1):53. doi: 10.1186/s13046-025-03289-8. J Exp Clin Cancer Res. 2025. PMID: 39953610 Free PMC article.
-
Uncovering somatic genetic drivers in prostate cancer through comprehensive genome-wide analysis.Geroscience. 2025 Jun;47(3):5039-5056. doi: 10.1007/s11357-025-01623-8. Epub 2025 Mar 29. Geroscience. 2025. PMID: 40156736 Free PMC article.
-
ACK1 condensates promote STAT5 signaling in lung squamous cell carcinoma.Cancer Cell Int. 2025 Jun 28;25(1):237. doi: 10.1186/s12935-025-03862-3. Cancer Cell Int. 2025. PMID: 40581662 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
