A quantitative biology approach correlates neuronal toxicity with the largest inclusions of TDP-43
- PMID: 35895809
- PMCID: PMC9328675
- DOI: 10.1126/sciadv.abm6376
A quantitative biology approach correlates neuronal toxicity with the largest inclusions of TDP-43
Abstract
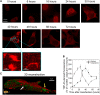
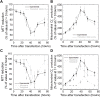
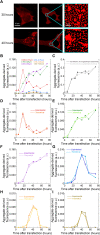
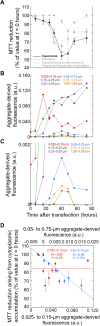
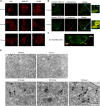
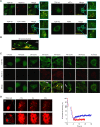
A number of neurodegenerative conditions are associated with the formation of cytosolic inclusions of TDP-43 within neurons. We expressed full-length TDP-43 in a motoneuron/neuroblastoma hybrid cell line (NSC-34) and exploited the high-resolution power of stimulated emission depletion microscopy to monitor the changes of nuclear and cytoplasmic TDP-43 levels and the formation of various size classes of cytoplasmic TDP-43 aggregates with time. Concomitantly, we monitored oxidative stress and mitochondrial impairment using the MitoSOX and MTT reduction assays, respectively. Using a quantitative biology approach, we attributed neuronal dysfunction associated with cytoplasmic deposition component to the formation of the largest inclusions, independently of stress granules. This is in contrast to other neurodegenerative diseases where toxicity is attributed to small oligomers. Using specific inhibitors, markers, and electron microscopy, the proteasome and autophagy were found to target mainly the largest deleterious inclusions, but their efficiency soon decreases without full recovery of neuronal viability.
Figures
References
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T., TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006). - PubMed
-
- Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., Feiden W., Kretzschmar H. A., Trojanowski J. Q., Lee V. M. Y., Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). - PubMed
-
- Mackenzie I. R., Bigio E. H., Ince P. G., Geser F., Neumann M., Cairns N. J., Kwong L. K., Forman M. S., Ravits J., Stewart H., Eisen A., McClusky L., Kretzschmar H. A., Monoranu C. M., Highley J. R., Kirby J., Siddique T., Shaw P. J., Lee V. M., Trojanowski J. Q., Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 61, 427–434 (2007). - PubMed
-
- Brettschneider J., Del Tredici K., Toledo J. B., Robinson J. L., Irwin D. J., Grossman M., Suh E., Van Deerlin V. M., Wood E. M., Baek Y., Kwong L., Lee E. B., Elman L., McCluskey L., Fang L., Feldengut S., Ludolph A. C., Lee V. M., Braak H., Trojanowski J. Q., Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 74, 20–38 (2013). - PMC - PubMed
-
- Hasegawa M., Arai T., Nonaka T., Kametani F., Yoshida M., Hashizume Y., Beach T. G., Buratti E., Baralle F., Morita M., Nakano I., Oda T., Tsuchiya K., Akiyama H., Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann. Neurol. 64, 60–70 (2008). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

