Supramolecular Complex of Photochromic Diarylethene and Cucurbit[7]uril: Fluorescent Photoswitching System for Biolabeling and Imaging
- PMID: 35895999
- PMCID: PMC9376957
- DOI: 10.1021/jacs.2c05036
Supramolecular Complex of Photochromic Diarylethene and Cucurbit[7]uril: Fluorescent Photoswitching System for Biolabeling and Imaging
Abstract
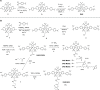
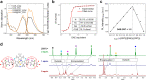
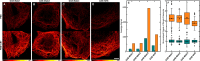
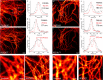
Photoswitchable fluorophores─proteins and synthetic dyes─whose emission is reversibly switched on and off upon illumination, are powerful probes for bioimaging, protein tracking, and super-resolution microscopy. Compared to proteins, synthetic dyes are smaller and brighter, but their photostability and the number of achievable switching cycles in aqueous solutions are lower. Inspired by the robust photoswitching system of natural proteins, we designed a supramolecular system based on a fluorescent diarylethene (DAE) and cucurbit[7]uril (CB7) (denoted as DAE@CB7). In this assembly, the photoswitchable DAE molecule is encapsulated by CB7 according to the host-guest principle, so that DAE is protected from the environment and its fluorescence brightness and fatigue resistance in pure water improved. The fluorescence quantum yield (Φfl) increased from 0.40 to 0.63 upon CB7 complexation. The photoswitching of the DAE@CB7 complex, upon alternating UV and visible light irradiations, can be repeated 2560 times in aqueous solution before half-bleaching occurs (comparable to fatigue resistance of the reversibly photoswitchable proteins), while free DAE can be switched on and off only 80 times. By incorporation of reactive groups [maleimide and N-hydroxysuccinimidyl (NHS) ester], we prepared bioconjugates of DAE@CB7 with antibodies and demonstrated both specific labeling of intracellular proteins in cells and the reversible on/off switching of the probes in cellular environments under irradiations with 355 nm/485 nm light. The bright emission and robust photoswitching of DAE-Male3@CB7 and DAE-NHS@CB7 complexes (without exclusion of air oxygen and addition of any stabilizing/antifading reagents) enabled confocal and super-resolution RESOLFT (reversible saturable optical fluorescence transitions) imaging with apparent 70-90 nm optical resolution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Supramolecular Complex of Cucurbit[7]uril with Diketopyrrolopyrole Dye: Fluorescence Boost, Biolabeling and Optical Microscopy.Angew Chem Int Ed Engl. 2024 Sep 2;63(36):e202410217. doi: 10.1002/anie.202410217. Epub 2024 Aug 2. Angew Chem Int Ed Engl. 2024. PMID: 38881490
-
Supramolecular Complexation of Quenched Rosamines with Cucurbit[7]Uril: Fluorescence Turn-ON Effect for Super-Resolution Imaging.J Am Chem Soc. 2025 Aug 13;147(32):28893-28902. doi: 10.1021/jacs.5c06406. Epub 2025 Jul 29. J Am Chem Soc. 2025. PMID: 40731377 Free PMC article.
-
Host-guest complexation between cucurbit[7]uril and doxepin induced supramolecular assembly.Org Biomol Chem. 2022 Jul 27;20(29):5796-5802. doi: 10.1039/d2ob01065h. Org Biomol Chem. 2022. PMID: 35833381
-
Cucurbit[8]uril-based supramolecular theranostics.J Nanobiotechnology. 2024 May 9;22(1):235. doi: 10.1186/s12951-024-02349-z. J Nanobiotechnology. 2024. PMID: 38725031 Free PMC article. Review.
-
Recent Progress of Photoswitchable Fluorescent Diarylethenes for Bioimaging.Chembiochem. 2024 Dec 2;25(23):e202400326. doi: 10.1002/cbic.202400326. Epub 2024 Nov 6. Chembiochem. 2024. PMID: 39235968 Review.
Cited by
-
Recent Advances in Photoswitchable Fluorescent and Colorimetric Probes.Molecules. 2024 May 27;29(11):2521. doi: 10.3390/molecules29112521. Molecules. 2024. PMID: 38893396 Free PMC article. Review.
-
Supramolecular Synthesis of Dithienylethene-Albumin Complexes for Enhanced Photoswitching In Photoacoustic Imaging-Guided Near-Infrared Photothermal Therapy.Small. 2025 Mar;21(12):e2409027. doi: 10.1002/smll.202409027. Epub 2025 Feb 26. Small. 2025. PMID: 40012339 Free PMC article.
-
Ligand-protected nanocluster-mediated photoswitchable fluorescent nanoprobes towards dual-color cellular imaging.Chem Sci. 2023 Aug 7;14(33):8823-8830. doi: 10.1039/d3sc03593j. eCollection 2023 Aug 23. Chem Sci. 2023. PMID: 37621438 Free PMC article.
-
No-wash fluorogenic labeling of proteins for reversible photoswitching in live cells.Chem Sci. 2023 Dec 18;15(4):1393-1401. doi: 10.1039/d3sc04953a. eCollection 2024 Jan 24. Chem Sci. 2023. PMID: 38274070 Free PMC article.
-
Amplifying dual-visible-light photoswitching in aqueous media via confinement promoted triplet-triplet energy transfer.Chem Sci. 2024 Mar 6;15(15):5539-5547. doi: 10.1039/d4sc00423j. eCollection 2024 Apr 17. Chem Sci. 2024. PMID: 38638239 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

