Humoral Response to the Third Dose of BNT162b2 COVID-19 Vaccine among Hemodialysis Patients
- PMID: 35896080
- PMCID: PMC9747736
- DOI: 10.1159/000525519
Humoral Response to the Third Dose of BNT162b2 COVID-19 Vaccine among Hemodialysis Patients
Abstract
Background: Hemodialysis patients are at high risk for severe COVID-19 disease. Despite a high early seropositivity rate, dialysis patients mount a dampened immune response following two doses of an mRNA vaccine. This study aimed to evaluate the serologic response to a booster dose of BNT162b2 vaccine, 6 months after the second dose, among hemodialysis patients.
Methods: This prospective study included 80 hemodialysis patients and 56 healthcare workers serving as controls. Serologic samples were evaluated before and ∼3 weeks after the third vaccine dose. The primary outcomes were the seropositivity rate and the log-transformed anti-SARS-COV-2 S1 (RBD) IgG as a continuous variable after the third dose. Secondary outcomes were the proportion of participants with "high response," defined as antibody levels >1,000 AU/mL, and "robust response," defined as antibody levels >4,160 AU/mL, according to prespecified cutoff values associated with neutralizing antibodies. Univariate and multivariate analyses were conducted to identify predictors of antibody response.
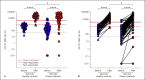
Results: Among 80 hemodialysis patients, seropositivity rates improved from 78% (62/80) before the third dose, up to 96% (77/80) after the booster dose. The S1-RBD log-transformed antibody level increased significantly following the third dose from 2.15 ± 0.75 to 3.99 ± 0.83 compared with 2.65 ± 0.4 to 4.31 ± 0.42 in the control group. Among the hemodialysis patients, 88% (70/80) became "high responders" (>1,000 AU/mL), and of these, 79% (63/80) mounted a "robust response" (>4,160 AU/mL). Baseline antibody level, dialysis therapy, and hypoalbuminemia were independent predictors of impaired antibody response.
Conclusions: A third dose of BNT162b2 COVID-19 vaccine, 6 months after the standard two-dose vaccination regimen, substantially improved humoral response in hemodialysis patients.
Keywords: Antibody response; COVID-19 vaccine; Hemodialysis patients; Third dose.
© 2022 S. Karger AG, Basel.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose. The results presented in this article have not been published previously in whole or part.
Figures
References
-
- Krueger KM, Ison MG, Ghossein C. Practical guide to vaccination in all stages of CKD, including patients treated by dialysis or kidney transplantation. Am J Kidney Dis. 2020;75((3)):417–425. - PubMed
-
- Rincon-Arevalo H, Choi M, Stefanski AL, Halleck F, Weber U, Szelinski F, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6((60)):eabj1031. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous