Maternal and perinatal obesity induce bronchial obstruction and pulmonary hypertension via IL-6-FoxO1-axis in later life
- PMID: 35896539
- PMCID: PMC9329333
- DOI: 10.1038/s41467-022-31655-z
Maternal and perinatal obesity induce bronchial obstruction and pulmonary hypertension via IL-6-FoxO1-axis in later life
Abstract
Obesity is a pre-disposing condition for chronic obstructive pulmonary disease, asthma, and pulmonary arterial hypertension. Accumulating evidence suggests that metabolic influences during development can determine chronic lung diseases (CLD). We demonstrate that maternal obesity causes early metabolic disorder in the offspring. Here, interleukin-6 induced bronchial and microvascular smooth muscle cell (SMC) hyperproliferation and increased airway and pulmonary vascular resistance. The key anti-proliferative transcription factor FoxO1 was inactivated via nuclear exclusion. These findings were confirmed using primary SMC treated with interleukin-6 and pharmacological FoxO1 inhibition as well as genetic FoxO1 ablation and constitutive activation. In vivo, we reproduced the structural and functional alterations in offspring of obese dams via the SMC-specific ablation of FoxO1. The reconstitution of FoxO1 using IL-6-deficient mice and pharmacological treatment did not protect against metabolic disorder but prevented SMC hyperproliferation. In human observational studies, childhood obesity was associated with reduced forced expiratory volume in 1 s/forced vital capacity ratio Z-score (used as proxy for lung function) and asthma. We conclude that the interleukin-6-FoxO1 pathway in SMC is a molecular mechanism by which perinatal obesity programs the bronchial and vascular structure and function, thereby driving CLD development. Thus, FoxO1 reconstitution provides a potential therapeutic option for preventing this metabolic programming of CLD.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



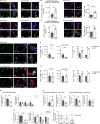

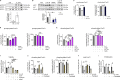


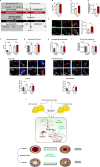
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

