Akkermansia muciniphila phospholipid induces homeostatic immune responses
- PMID: 35896748
- PMCID: PMC9328018
- DOI: 10.1038/s41586-022-04985-7
Akkermansia muciniphila phospholipid induces homeostatic immune responses
Abstract
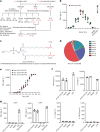
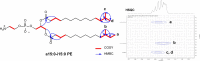
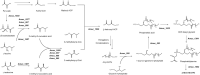
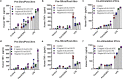
Multiple studies have established associations between human gut bacteria and host physiology, but determining the molecular mechanisms underlying these associations has been challenging1-3. Akkermansia muciniphila has been robustly associated with positive systemic effects on host metabolism, favourable outcomes to checkpoint blockade in cancer immunotherapy and homeostatic immunity4-7. Here we report the identification of a lipid from A. muciniphila's cell membrane that recapitulates the immunomodulatory activity of A. muciniphila in cell-based assays8. The isolated immunogen, a diacyl phosphatidylethanolamine with two branched chains (a15:0-i15:0 PE), was characterized through both spectroscopic analysis and chemical synthesis. The immunogenic activity of a15:0-i15:0 PE has a highly restricted structure-activity relationship, and its immune signalling requires an unexpected toll-like receptor TLR2-TLR1 heterodimer9,10. Certain features of the phospholipid's activity are worth noting: it is significantly less potent than known natural and synthetic TLR2 agonists; it preferentially induces some inflammatory cytokines but not others; and, at low doses (1% of EC50) it resets activation thresholds and responses for immune signalling. Identifying both the molecule and an equipotent synthetic analogue, its non-canonical TLR2-TLR1 signalling pathway, its immunomodulatory selectivity and its low-dose immunoregulatory effects provide a molecular mechanism for a model of A. muciniphila's ability to set immunological tone and its varied roles in health and disease.
© 2022. The Author(s).
Conflict of interest statement
Four of the authors (M.B., C.D.C., R.J.X. and J.C.) have submitted a patent application for materials described in this manuscript. R.J.X. is co-founder of Jnana Therapeutics and Celsius Therapeutics and is a consultant to Nestle; these organizations had no roles in this study. All other authors declare no competing interests.
Figures






References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

