A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Efficacy and Safety of Viloxazine Extended-Release Capsules in Adults with Attention-Deficit/Hyperactivity Disorder
- PMID: 35896943
- PMCID: PMC9328182
- DOI: 10.1007/s40263-022-00938-w
A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Efficacy and Safety of Viloxazine Extended-Release Capsules in Adults with Attention-Deficit/Hyperactivity Disorder
Abstract
Background and objective: Attention-deficit/hyperactivity disorder is a neurodevelopmental disorder that typically begins in childhood and often persists into adulthood. Recent phase III trials have demonstrated the efficacy and safety of viloxazine extended-release capsules (viloxazine ER; Qelbree®) in pediatrics (6-17 years of age). The aim of this study was to evaluate the efficacy and safety of viloxazine ER in adults with attention-deficit/hyperactivity disorder.
Methods: This was a phase III, randomized, double-blind, placebo-controlled, two-arm trial in adults (18-65 years of age) with attention-deficit/hyperactivity disorder. Eligible subjects were randomized 1:1 to viloxazine ER (flexible dose of 200-600 mg/day) or matched placebo. The primary efficacy endpoint was the change from baseline at end of study (week 6) in the Adult ADHD Investigator Symptom Rating Scale (AISRS) total score. The key secondary endpoint was the change from baseline at end of study in the Clinical Global Impressions-Severity of Illness (CGI-S) score. Additional secondary outcome measures included the AISRS Inattention and Hyperactivity/Impulsivity subscales, the Behavior Rating Inventory of Executive Function-Adult (BRIEF-A), the Generalized Anxiety Disorder-7 Item (GAD-7), and the Clinical Global Impressions-Improvement (CGI-I); each was analyzed at end of study. Responder rates on CGI scales and the AISRS were also assessed.
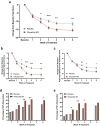
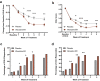
Results: A total of 374 subjects were randomized. At end of study, the mean viloxazine ER dose was 504 mg. The reduction in the change from baseline at end of study AISRS total score (least-square means ± standard error) was significantly greater in subjects treated with viloxazine ER (-15.5 ± 0.91) compared with placebo (-11.7 ± 0.90), p = 0.0040. The reduction in the CGI-S score was also significantly greater in subjects treated with viloxazine ER (-1.4 ± 0.10) compared with placebo (-1.0 ± 0.10), p = 0.0023. The viloxazine ER group demonstrated significantly greater improvements in the AISRS Inattention (p = 0.0015) and Hyperactivity/Impulsivity (p = 0.0380) subscales, the CGI-I (p = 0.0076), and the BRIEF-A Global Executive Composite (p = 0.0468) and Metacognition Index (p = 0.0100). Analysis of categorical secondary endpoints revealed that the viloxazine ER group had a significantly higher AISRS 30% response rate compared with placebo (p = 0.0395); all other comparisons were not significant. Many treatment effects (including the primary and key secondary endpoints) were significant by week 2. The most common treatment-related adverse events that occurred in ≥5% of subjects receiving viloxazine ER were insomnia (14.8%), fatigue (11.6%), nausea (10.1%), decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). Viloxazine ER was well tolerated, with a 9.0% discontinuation rate due to adverse events compared with 4.9% in the placebo group.
Conclusions: Treatment with viloxazine ER resulted in a statistically significant improvement in primary and key secondary endpoints, indicating improvements in attention-deficit/hyperactivity disorder symptomology, executive function, and overall clinical illness severity in adults. Viloxazine ER was well tolerated at the tested doses in adults with attention-deficit/hyperactivity disorder.
Clinical trial registration: Clinicaltrials.gov identifier: NCT04016779.
Plain language summary
Attention-deficit/hyperactivity disorder (ADHD) is a condition characterized by inattention (difficulty maintaining focus), and/or impulsiveness/hyperactivity. In 2021, a nonstimulant medication called viloxazine ER (brand name: Qelbree®) received US FDA-approval for ADHD in children and adolescents (aged 6 to 17 years), based on efficacy and safety demonstrated in clinical trials. Here we present results of a phase 3, randomized, double-blind, placebo-controlled, clinical trial that enrolled 374 adults with ADHD. In this trial, half the patients received viloxazine ER, and half received placebo (identical capsule without active ingredient). Medication doses ranged from 200–600 mg/day, based on symptom response and presence of side effects. To reduce bias, patients and investigators did not know which medication the patient was receiving. The primary measure of efficacy was the Adult ADHD Investigator Symptom Rating Scale (AISRS), a standardized questionnaire rating presence and severity of patient-reported ADHD symptoms. At the end of the 6-week trial, participants receiving viloxazine ER showed greater improvement in ADHD symptoms according to AISRS than those receiving placebo. Improvement was seen in both the Inattentive and Impulsive/Hyperactive components of ADHD and in other study measures, including a measure of behaviors called Executive Function. Viloxazine ER was generally safe and well-tolerated in the trial. The most common side effects were insomnia (14.8%), fatigue (11.6%), and nausea (10.1%). Overall, 9.0% of patients receiving viloxazine and 5% receiving placebo left the trial because of side effects. Due to these positive results, the US FDA recently approved viloxazine ER to treat adults with ADHD.
© 2022. The Author(s).
Conflict of interest statement
AN, JTH, TL, NF, and JR, are employees of Supernus Pharmaceuticals, Inc. SAC, OO, ARK, and SS were employees of Supernus Pharmaceuticals, Inc. at the time of this work. AJC has received research support from Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Ironshore Pharmaceuticals, Janssen, KemPharm, Lundbeck, Neos Therapeutics (now Aytu BioPharma), Neurovance, Noven, Otsuka, Purdue Canada, Rhodes Pharmaceuticals, Shire, Sunovion, Supernus Pharmaceuticals, Inc., Takeda, and Tris Pharma. He has received fees for consulting, advisory boards, and promotional speaking from Adlon Therapeutics, Aevi Genomics, Akili Interactive, Arbor Pharmaceuticals, Atentiv, Cingulate Therapeutics, Corium, Ironshore Pharmaceuticals, Janssen, Lundbeck, MedAvante-ProPhase, Neos Therapeutics (now Aytu BioPharma), NLS Pharmaceutics, Noven, Otsuka, Shire, Sunovion, Supernus Pharmaceuticals, Inc., Takeda, and Tris Pharma. He is an employee and board member of the Neuroscience Education Institute. AC has received research support from, served as a consultant or speaker for, or served on an advisory board for Allergan, Takeda (Shire), Emalex, Pearson, Akili, Arbor, Cingulate Therapeutics, Ironshore, Lumos, Aevi Genomic Medicine, Neos Therapeutics, Neurovance, Noven, Otsuka, Pfizer, Purdue, Adlon, Rhodes, Sunovion, Tris, KemPharm, Supernus Pharmaceuticals, Inc., US Food and Drug Administration, NLS Pharma, and Jazz.
Figures

Comment in
-
Authors' Reply to Singh and Balasundaram: Comment on "A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Efficacy and Safety of Viloxazine Extended-Release Capsules in Adults with Attention-Deficit/Hyperactivity Disorder".CNS Drugs. 2022 Dec;36(12):1333-1335. doi: 10.1007/s40263-022-00967-5. Epub 2022 Nov 4. CNS Drugs. 2022. PMID: 36331781 Free PMC article. No abstract available.
-
Comment on: "A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial Assessing the Efficacy and Safety of Viloxazine Extended-Release Capsules in Adults with Attention Deficit/Hyperactivity Disorder".CNS Drugs. 2022 Dec;36(12):1331-1332. doi: 10.1007/s40263-022-00966-6. Epub 2022 Nov 4. CNS Drugs. 2022. PMID: 36331782 No abstract available.
References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Publishing; 2013.
-
- Weiss G, Hechtman LT. Hyperactive children grown up: ADHD in children, adolescents, and adults. 2. New York: Guilford Press; 1993.
-
- Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816–818. - PubMed
-
- Turgay A, Goodman DW, Asherson P, Lasser RA, Babcock TF, Pucci ML, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192–201. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

