Deletion of Abi3/Gngt2 influences age-progressive amyloid β and tau pathologies in distinctive ways
- PMID: 35897046
- PMCID: PMC9327202
- DOI: 10.1186/s13195-022-01044-1
Deletion of Abi3/Gngt2 influences age-progressive amyloid β and tau pathologies in distinctive ways
Abstract
Background: The S209F variant of Abelson Interactor Protein 3 (ABI3) increases risk for Alzheimer's disease (AD), but little is known about its function in relation to AD pathogenesis.
Methods: Here, we use a mouse model that is deficient in Abi3 locus to study how the loss of function of Abi3 impacts two cardinal neuropathological hallmarks of AD-amyloid β plaques and tau pathology. Our study employs extensive neuropathological and transcriptomic characterization using transgenic mouse models and adeno-associated virus-mediated gene targeting strategies.
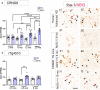
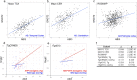
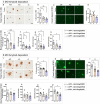
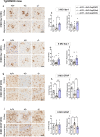
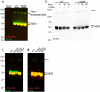
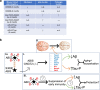
Results: Analysis of bulk RNAseq data confirmed age-progressive increase in Abi3 levels in rodent models of AD-type amyloidosis and upregulation in AD patients relative to healthy controls. Using RNAscope in situ hybridization, we localized the cellular distribution of Abi3 in mouse and human brains, finding that Abi3 is expressed in both microglial and non-microglial cells. Next, we evaluated Abi3-/- mice and document that both Abi3 and its overlapping gene, Gngt2, are disrupted in these mice. Using multiple transcriptomic datasets, we show that expression of Abi3 and Gngt2 are tightly correlated in rodent models of AD and human brains, suggesting a tight co-expression relationship. RNAseq of the Abi3-Gngt2-/- mice revealed upregulation of Trem2, Plcg2, and Tyrobp, concomitant with induction of an AD-associated neurodegenerative signature, even in the absence of AD-typical neuropathology. In APP mice, loss of Abi3-Gngt2 resulted in a gene dose- and age-dependent reduction in Aβ deposition. Additionally, in Abi3-Gngt2-/- mice, expression of a pro-aggregant form of human tau exacerbated tauopathy and astrocytosis. Further, using in vitro culture assays, we show that the AD-associated S209F mutation alters the extent of ABI3 phosphorylation.
Conclusions: These data provide an important experimental framework for understanding the role of Abi3-Gngt2 function and early inflammatory gliosis in AD. Our studies also demonstrate that inflammatory gliosis could have opposing effects on amyloid and tau pathology, highlighting the unpredictability of targeting immune pathways in AD.
Keywords: Alzheimer’s disease; Disease signature; Gene dose; Neurofibrillary tangle; Plaque burden; Risk factor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- U01 AG046152/AG/NIA NIH HHS/United States
- R01 AG023571/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- R01 AG030146/AG/NIA NIH HHS/United States
- R01 AG032990/AG/NIA NIH HHS/United States
- R01 NS080820/NS/NINDS NIH HHS/United States
- RC2 AG036547/AG/NIA NIH HHS/United States
- P01 AG017216/AG/NIA NIH HHS/United States
- R01 AG018023/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- R01 AG039495/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- R01 AG036042/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- RF1 AG057473/AG/NIA NIH HHS/United States
- U01 AG046139/AG/NIA NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- U01 AG046161/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01 AG048015/AG/NIA NIH HHS/United States
- P50 AG025711/AG/NIA NIH HHS/United States
- U01 AG006786/AG/NIA NIH HHS/United States
- R01 AG036836/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

