Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice
- PMID: 35897643
- PMCID: PMC9332143
- DOI: 10.3390/ijms23158066
Possible Role of GnIH as a Novel Link between Hyperphagia-Induced Obesity-Related Metabolic Derangements and Hypogonadism in Male Mice
Abstract
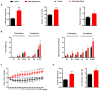
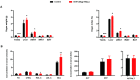
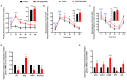
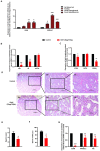
Gonadotropin-inhibitory hormone (GnIH) is a reproductive inhibitor and an endogenous orexigenic neuropeptide that may be involved in energy homeostasis and reproduction. However, whether GnIH is a molecular signal link of metabolism and the reproductive system, and thus, regulates reproductive activity as a function of the energy state, is still unknown. In the present study, we investigated the involvement of GnIH in glycolipid metabolism and reproduction in vivo, and in the coupling between these two processes in the testis level. Our results showed that chronic intraperitoneal injection of GnIH into male mice not only increased food intake and altered meal microstructure but also significantly elevated body mass due to the increased mass of liver and epididymal white adipose tissue (eWAT), despite the loss of testicular weight. Furthermore, chronic intraperitoneal administration of GnIH to male mice resulted in obesity-related glycolipid metabolic derangements, showing hyperlipidemia, hyperglycemia, glucose intolerance, and insulin resistance through changes in the expression of glucose and lipid metabolism-related genes in the pancreas and eWAT, respectively. Interestingly, the expression of GnIH and GPR147 was markedly increased in the testis of mice under conditions of energy imbalance, such as fasting, acute hypoglycemia, and hyperglycemia. In addition, chronic GnIH injection markedly inhibited glucose and lipid metabolism of mice testis while significantly decreasing testosterone synthesis and sperm quality, inducing hypogonadism. These observations indicated that orexigenic GnIH triggers hyperphagia-induced obesity-related metabolic derangements and hypogonadism in male mice, suggesting that GnIH is an emerging candidate for coupling metabolism and fertility by involvement in obesity and metabolic disorder-induced reproductive dysfunction of the testes.
Keywords: GnIH; food intake; glucose and lipid metabolism; reproduction; testis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hart R.J., Doherty D.A., Mori T.A., Adams L.A., Huang R.C., Minaee N., Handelsman D.J., McLachlan R., Norman R.J., Dickinson J.E., et al. Features of the metabolic syndrome in late adolescence are associated with impaired testicular function at 20 years of age. Hum. Reprod. 2019;34:389–402. doi: 10.1093/humrep/dey371. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

