Three-Dimensional Culture of Cartilage Tissue on Nanogel-Cross-Linked Porous Freeze-Dried Gel Scaffold for Regenerative Cartilage Therapy: A Vibrational Spectroscopy Evaluation
- PMID: 35897669
- PMCID: PMC9332688
- DOI: 10.3390/ijms23158099
Three-Dimensional Culture of Cartilage Tissue on Nanogel-Cross-Linked Porous Freeze-Dried Gel Scaffold for Regenerative Cartilage Therapy: A Vibrational Spectroscopy Evaluation
Abstract
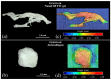
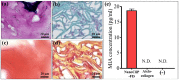
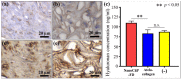
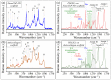
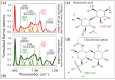
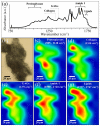
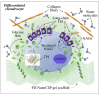
This study presents a set of vibrational characterizations on a nanogel-cross-linked porous freeze-dried gel (NanoCliP-FD gel) scaffold for tissue engineering and regenerative therapy. This scaffold is designed for the in vitro culture of high-quality cartilage tissue to be then transplanted in vivo to enable recovery from congenital malformations in the maxillofacial area or crippling jaw disease. The three-dimensional scaffold for in-plate culture is designed with interface chemistry capable of stimulating cartilage formation and maintaining its structure through counteracting the dedifferentiation of mesenchymal stem cells (MSCs) during the formation of cartilage tissue. The developed interface chemistry enabled high efficiency in both growth rate and tissue quality, thus satisfying the requirements of large volumes, high matrix quality, and superior mechanical properties needed in cartilage transplants. We characterized the cartilage tissue in vitro grown on a NanoCliP-FD gel scaffold by human periodontal ligament-derived stem cells (a type of MSC) with cartilage grown by the same cells and under the same conditions on a conventional (porous) atelocollagen scaffold. The cartilage tissues produced by the MSCs on different scaffolds were comparatively evaluated by immunohistochemical and spectroscopic analyses. Cartilage differentiation occurred at a higher rate when MSCs were cultured on the NanoCliP-FD gel scaffold compared to the atelocollagen scaffold, and produced a tissue richer in cartilage matrix. In situ spectroscopic analyses revealed the cell/scaffold interactive mechanisms by which the NanoCliP-FD gel scaffold stimulated such increased efficiency in cartilage matrix formation. In addition to demonstrating the high potential of human periodontal ligament-derived stem cell cultures on NanoCliP-FD gel scaffolds in regenerative cartilage therapy, the present study also highlights the novelty of Raman spectroscopy as a non-destructive method for the concurrent evaluation of matrix quality and cell metabolic response. In situ Raman analyses on living cells unveiled for the first time the underlying physiological mechanisms behind such improved chondrocyte performance.
Keywords: NanoCliP-FD gel scaffold; human periodontal ligament-derived stem cell; in situ Raman spectroscopy; regenerative therapy; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Development of a skeletal muscle sheet with direct reprogramming-induced myoblasts on a nanogel-cross-linked porous freeze-dried gel scaffold in a mouse gastroschisis model.Pediatr Surg Int. 2024 Aug 26;40(1):241. doi: 10.1007/s00383-024-05811-z. Pediatr Surg Int. 2024. PMID: 39183231
-
Cholesterol-Bearing Polysaccharide-Based Nanogels for Development of Novel Immunotherapy and Regenerative Medicine.Gels. 2024 Mar 18;10(3):206. doi: 10.3390/gels10030206. Gels. 2024. PMID: 38534624 Free PMC article. Review.
-
Osteogenic response of mesenchymal progenitor cells to natural polysaccharide nanogel and atelocollagen scaffolds: A spectroscopic study.Mater Sci Eng C Mater Biol Appl. 2019 Jun;99:1325-1340. doi: 10.1016/j.msec.2019.02.043. Epub 2019 Feb 21. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30889667
-
In Vivo Regeneration of Large Bone Defects by Cross-Linked Porous Hydrogel: A Pilot Study in Mice Combining Micro Tomography, Histological Analyses, Raman Spectroscopy and Synchrotron Infrared Imaging.Materials (Basel). 2020 Sep 25;13(19):4275. doi: 10.3390/ma13194275. Materials (Basel). 2020. PMID: 32992758 Free PMC article.
-
Scaffold: a novel carrier for cell and drug delivery.Crit Rev Ther Drug Carrier Syst. 2012;29(1):1-63. doi: 10.1615/critrevtherdrugcarriersyst.v29.i1.10. Crit Rev Ther Drug Carrier Syst. 2012. PMID: 22356721 Review.
Cited by
-
Spectroscopic Analysis of the Extracellular Matrix in Naked Mole-Rat Temporomandibular Joints.Gels. 2025 May 30;11(6):414. doi: 10.3390/gels11060414. Gels. 2025. PMID: 40558714 Free PMC article.
-
Sericin promotes chondrogenic proliferation and differentiation via glycolysis and Smad2/3 TGF-β signaling inductions and alleviates inflammation in three-dimensional models.Sci Rep. 2024 May 21;14(1):11553. doi: 10.1038/s41598-024-62516-y. Sci Rep. 2024. PMID: 38773312 Free PMC article.
-
Development of a skeletal muscle sheet with direct reprogramming-induced myoblasts on a nanogel-cross-linked porous freeze-dried gel scaffold in a mouse gastroschisis model.Pediatr Surg Int. 2024 Aug 26;40(1):241. doi: 10.1007/s00383-024-05811-z. Pediatr Surg Int. 2024. PMID: 39183231
-
Exploring the Potential of Nanogels: From Drug Carriers to Radiopharmaceutical Agents.Adv Healthc Mater. 2024 Jan;13(1):e2301404. doi: 10.1002/adhm.202301404. Epub 2023 Sep 28. Adv Healthc Mater. 2024. PMID: 37717209 Free PMC article. Review.
-
Cholesterol-Bearing Polysaccharide-Based Nanogels for Development of Novel Immunotherapy and Regenerative Medicine.Gels. 2024 Mar 18;10(3):206. doi: 10.3390/gels10030206. Gels. 2024. PMID: 38534624 Free PMC article. Review.
References
-
- Hubka K.M., Dahlin R.L., Meretoja V.V., Kasper F.K., Mikos A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: Monoculture and coculture of articular chondrocytes and mesenchymal stemm cells. Tissue Eng. Part B. 2014;20:641–654. doi: 10.1089/ten.teb.2014.0034. - DOI - PMC - PubMed

