A microRNA Signature for the Diagnosis of Statins Intolerance
- PMID: 35897722
- PMCID: PMC9330734
- DOI: 10.3390/ijms23158146
A microRNA Signature for the Diagnosis of Statins Intolerance
Abstract
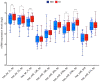
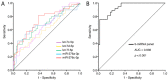
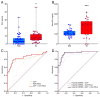
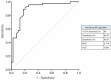
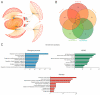
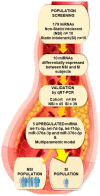
Atherosclerotic cardiovascular diseases (ASCVD) are the leading cause of morbidity and mortality in Western societies. Statins are the first-choice therapy for dislipidemias and are considered the cornerstone of ASCVD. Statin-associated muscle symptoms are the main reason for dropout of this treatment. There is an urgent need to identify new biomarkers with discriminative precision for diagnosing intolerance to statins (SI) in patients. MicroRNAs (miRNAs) have emerged as evolutionarily conserved molecules that serve as reliable biomarkers and regulators of multiple cellular events in cardiovascular diseases. In the current study, we evaluated plasma miRNAs as potential biomarkers to discriminate between the SI vs. non-statin intolerant (NSI) population. It is a multicenter, prospective, case-control study. A total of 179 differentially expressed circulating miRNAs were screened in two cardiovascular risk patient cohorts (high and very high risk): (i) NSI (n = 10); (ii) SI (n = 10). Ten miRNAs were identified as being overexpressed in plasma and validated in the plasma of NSI (n = 45) and SI (n = 39). Let-7c-5p, let-7d-5p, let-7f-5p, miR-376a-3p and miR-376c-3p were overexpressed in the plasma of SI patients. The receiver operating characteristic curve analysis supported the discriminative potential of the diagnosis. We propose a three-miRNA predictive fingerprint (let-7f, miR-376a-3p and miR-376c-3p) and several clinical variables (non-HDLc and years of dyslipidemia) for SI discrimination; this model achieves sensitivity, specificity and area under the receiver operating characteristic curve (AUC) of 83.67%, 88.57 and 89.10, respectively. In clinical practice, this set of miRNAs combined with clinical variables may discriminate between SI vs. NSI subjects. This multiparametric model may arise as a potential diagnostic biomarker with clinical value.
Keywords: atherosclerotic cardiovascular diseases; biomarkers; circulating microRNAs; statin intolerance; statins-adverse myalgia symptoms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. ESC Scientific Document Group (2020). 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. - DOI - PubMed
-
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C., Bhala N., Peto R., Barnes E.H., Keech A., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 0048-2017/the European Regional Development Fund (ERDF) Integrated Territorial Initiative (ITI PI0048-2017
- ITI0017-2019/the European Regional Development Fund (ERDF) Integrated Territorial Initiative
- (PEER 0019-2020)/the European Regional Development Fund (ERDF) and Rare Diseases Strategic Project
- 0012-2019/Spanish Society of Cardiology
- 0013_2019/Plan Propio de INIBICA

