The Pt(S-pr-thiosal)2 and BCL1 Leukemia Lymphoma: Antitumor Activity In Vitro and In Vivo
- PMID: 35897737
- PMCID: PMC9332548
- DOI: 10.3390/ijms23158161
The Pt(S-pr-thiosal)2 and BCL1 Leukemia Lymphoma: Antitumor Activity In Vitro and In Vivo
Abstract
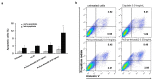
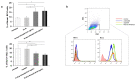
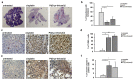
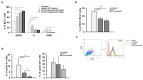
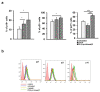
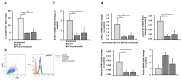
B cell malignancies are, despite the development of targeted therapy in a certain percentage of the patients still a chronic disease with relapses, requiring multiple lines of therapy. Regimens that include platinum-based drugs provide high response rates in different B cell lymphomas, high-risk chronic lymphocytic leukemia (CLL), and devastating complication of CLL, Richter's syndrome. The aim of this study was to explore the potential antitumor activity of previously synthetized platinum(IV) complex with alkyl derivatives of thyosalicilc acid, PtCl2(S-pr-thiosal)2, toward murine BCL1 cells and to delineate possible mechanisms of action. The PtCl2(S-pr-thiosal)2 reduced the viability of BCL1 cells in vitro but also reduced the growth of metastases in the leukemia lymphoma model in BALB/c mice. PtCl2(S-pr-thiosal)2 induced apoptosis, inhibited proliferation of BCL1 cells, and induced cell cycle disturbance. Treatment of BCL1 cells with PtCl2(S-pr-thiosal)2 inhibited expression of cyclin D3 and cyclin E and enhanced expression of cyclin-dependent kinase inhibitors p16, p21, and p27 resulting in cell cycle arrest in the G1 phase, reduced the percentage of BCL1 cells in the S phase, and decreased expression of Ki-67. PtCl2(S-pr-thiosal)2 treatment reduced expression of phosphorylated STAT3 and downstream-regulated molecules associated with cancer stemness and proliferation, NANOG, cyclin D3, and c-Myc, and expression of phosphorylated NFκB in vitro and in vivo. In conclusion, PtCl2(S-pr-thiosal)2 reduces STAT3 and NFκB phosphorylation resulting in inhibition of BCL1 cell proliferation and the triggering of apoptotic cell death.
Keywords: BCL1; Bcl-2; Pt(S-pr-thiosal)2 complex; STAT3; antitumor activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Yao Y., Lin X., Li F., Jin J., Wang H. The global burden and attributable risk factors of chronic lymphocytic leukemia in 204 countries and territories from 1990 to 2019: Analysis based on the global burden of disease study 2019. Biomed. Eng. Online. 2022;21:4. doi: 10.1186/s12938-021-00973-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

