SaRCC1, a Regulator of Chromosome Condensation 1 (RCC1) Family Protein Gene from Spartina alterniflora, Negatively Regulates Salinity Stress Tolerance in Transgenic Arabidopsis
- PMID: 35897748
- PMCID: PMC9332369
- DOI: 10.3390/ijms23158172
SaRCC1, a Regulator of Chromosome Condensation 1 (RCC1) Family Protein Gene from Spartina alterniflora, Negatively Regulates Salinity Stress Tolerance in Transgenic Arabidopsis
Abstract
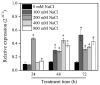
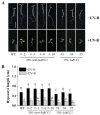
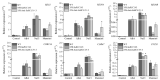
A regulator of chromosome condensation 1 (RCC1) family protein has been functionally characterized to be involved in various cellular processes. In this study, one RCC1 gene named SaRCC1 was cloned from the full-length cDNA library of Spartinaalterniflora. The open reading frame (ORF) of SaRCC1 was 1440 bp, and it encoded 479 amino acids with a calculated molecular mass of 51.65 kDa. Multiple amino acid sequence alignments showed that SaRCC1 had high identity with other plant RCC1s, and the phylogenetic analysis indicated that SaRCC1 had a closer affinity to Zea mays RCC1 family protein (ZmRCC1). SaRCC1 gene was induced under salt stress conditions, and its encoded protein was located in peroxisome. In order to further investigate the function of SaRCC1, transgenic Arabidopsis plants ectopically both sense-overexpressing and antisense-overexpressing SaRCC1 were generated. SaRCC1-overexpressing lines exhibited an increased salt and ABA hypersensitivity and reduced resistance to salinity stress. On the other hand, the transcripts of some stress-responsive genes in the SaRCC1 transgenic plants were affected in response to salinity stress. Our results provide evidence for the involvement of SaRCC1, negatively regulating salt stress responses by affecting stress-related gene expression in Arabidopsis.
Keywords: Spartina alterniflora; UVR8; regulator of chromosome condensation 1; salinity stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A stress inducible SUMO conjugating enzyme gene (SaSce9) from a grass halophyte Spartina alterniflora enhances salinity and drought stress tolerance in Arabidopsis.BMC Plant Biol. 2012 Oct 10;12:187. doi: 10.1186/1471-2229-12-187. BMC Plant Biol. 2012. PMID: 23051937 Free PMC article.
-
Overexpression of HbMBF1a, encoding multiprotein bridging factor 1 from the halophyte Hordeum brevisubulatum, confers salinity tolerance and ABA insensitivity to transgenic Arabidopsis thaliana.Plant Mol Biol. 2020 Jan;102(1-2):1-17. doi: 10.1007/s11103-019-00926-7. Epub 2019 Oct 26. Plant Mol Biol. 2020. PMID: 31655970 Free PMC article.
-
The bamboo aquaporin gene PeTIP4;1-1 confers drought and salinity tolerance in transgenic Arabidopsis.Plant Cell Rep. 2017 Apr;36(4):597-609. doi: 10.1007/s00299-017-2106-3. Epub 2017 Feb 6. Plant Cell Rep. 2017. PMID: 28168515
-
Overexpression of a nascent polypeptide associated complex gene (SaβNAC) of Spartina alterniflora improves tolerance to salinity and drought in transgenic Arabidopsis.Biochem Biophys Res Commun. 2012 Aug 10;424(4):747-52. doi: 10.1016/j.bbrc.2012.07.023. Epub 2012 Jul 15. Biochem Biophys Res Commun. 2012. PMID: 22809508
-
Plant NHX Antiporters: From Function to Biotechnological Application, with Case Study.Curr Protein Pept Sci. 2021;22(1):60-73. doi: 10.2174/1389203721666201103085151. Curr Protein Pept Sci. 2021. PMID: 33143624 Review.
Cited by
-
Genome-wide characterization of regulator of chromosome condensation 1 (RCC1) gene family in Artemisia annua L. revealed a conservation evolutionary pattern.BMC Genomics. 2023 Nov 18;24(1):692. doi: 10.1186/s12864-023-09786-4. BMC Genomics. 2023. PMID: 37980503 Free PMC article.
-
Recent allopolyploidization and transcriptomic asymmetry in the mangrove shrub Acanthus tetraploideus.BMC Genomics. 2025 May 2;26(1):438. doi: 10.1186/s12864-025-11557-2. BMC Genomics. 2025. PMID: 40316933 Free PMC article.
-
Genome-wide identification and expression analysis of the regulator of chromosome condensation 1 gene family in wheat (Triticum aestivum L.).Front Plant Sci. 2023 Feb 24;14:1124905. doi: 10.3389/fpls.2023.1124905. eCollection 2023. Front Plant Sci. 2023. PMID: 36909424 Free PMC article.
-
Exploring Novel Genomic Loci and Candidate Genes Associated with Plant Height in Bulgarian Bread Wheat via Multi-Model GWAS.Plants (Basel). 2024 Oct 3;13(19):2775. doi: 10.3390/plants13192775. Plants (Basel). 2024. PMID: 39409644 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

