Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis
- PMID: 35897760
- PMCID: PMC9329713
- DOI: 10.3390/ijms23158183
Nanodiamonds as Possible Tools for Improved Management of Bladder Cancer and Bacterial Cystitis
Abstract
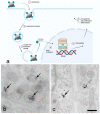
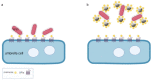
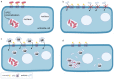
Nanodiamonds (NDs) are a class of carbon nanomaterials with sizes ranging from a few nm to micrometres. Due to their excellent physical, chemical and optical properties, they have recently attracted much attention in biomedicine. In addition, their exceptional biocompatibility and the possibility of precise surface functionalisation offer promising opportunities for biological applications such as cell labelling and imaging, as well as targeted drug delivery. However, using NDs for selective targeting of desired biomolecules within a complex biological system remains challenging. Urinary bladder cancer and bacterial cystitis are major diseases of the bladder with high incidence and poor treatment options. In this review, we present: (i) the synthesis, properties and functionalisation of NDs; (ii) recent advances in the study of various NDs used for better treatment of bladder cancer and (iii) bacterial cystitis; and (iv) the use of NDs in theranostics of these diseases.
Keywords: bacterial cystitis; bladder cancer; functionalisation; nanodiamonds; urinary bladder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

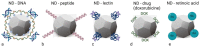
Similar articles
-
Production, surface modification and biomedical applications of nanodiamonds: A sparkling tool for theranostics.Mater Sci Eng C Mater Biol Appl. 2019 Apr;97:913-931. doi: 10.1016/j.msec.2018.12.073. Epub 2018 Dec 28. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30678981 Review.
-
Nanodiamonds: A Cutting-Edge Approach to Enhancing Biomedical Therapies and Diagnostics in Biosensing.Chem Rec. 2024 Apr;24(4):e202400006. doi: 10.1002/tcr.202400006. Epub 2024 Mar 26. Chem Rec. 2024. PMID: 38530037 Review.
-
Nanodiamonds for bioapplications-specific targeting strategies.Biochim Biophys Acta Gen Subj. 2020 Feb;1864(2):129354. doi: 10.1016/j.bbagen.2019.04.019. Epub 2019 May 7. Biochim Biophys Acta Gen Subj. 2020. PMID: 31071412 Review.
-
Nanodiamonds facilitate killing of intracellular uropathogenic E. coli in an in vitro model of urinary tract infection pathogenesis.PLoS One. 2018 Jan 11;13(1):e0191020. doi: 10.1371/journal.pone.0191020. eCollection 2018. PLoS One. 2018. PMID: 29324795 Free PMC article.
-
Nanodiamonds as novel nanomaterials for biomedical applications: drug delivery and imaging systems.Int J Nanomedicine. 2013;8:203-20. doi: 10.2147/IJN.S37348. Epub 2013 Jan 9. Int J Nanomedicine. 2013. PMID: 23326195 Free PMC article. Review.
Cited by
-
Nanoparticle-Based Techniques for Bladder Cancer Imaging: A Review.Int J Mol Sci. 2023 Feb 14;24(4):3812. doi: 10.3390/ijms24043812. Int J Mol Sci. 2023. PMID: 36835222 Free PMC article. Review.
-
Interaction of Nanomaterials with Cells and Tissues.Int J Mol Sci. 2023 Sep 4;24(17):13667. doi: 10.3390/ijms241713667. Int J Mol Sci. 2023. PMID: 37686473 Free PMC article.
-
A Novel Approach for Bladder Cancer Treatment: Nanoparticles as a Drug Delivery System.Int J Nanomedicine. 2024 Dec 17;19:13461-13483. doi: 10.2147/IJN.S498729. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39713223 Free PMC article. Review.
-
Does Selection for Longevity in Acheta domesticus Involve Sirtuin Activity Modulation and Differential Response to Activators (Resveratrol and Nanodiamonds)?Int J Mol Sci. 2024 Jan 22;25(2):1329. doi: 10.3390/ijms25021329. Int J Mol Sci. 2024. PMID: 38279331 Free PMC article.
-
Nanodiamonds: Next generation nano-theranostics for cancer therapy.Cancer Lett. 2024 Apr 10;587:216710. doi: 10.1016/j.canlet.2024.216710. Epub 2024 Feb 16. Cancer Lett. 2024. PMID: 38369006 Free PMC article. Review.
References
-
- Volkov K.V., Danilenko V.V., Elin V.I. Synthesis of diamond from the carbon in the detonation products of explosives. Combust. Explos. Shock. Waves. 1990;26:366–368. doi: 10.1007/BF00751383. - DOI
-
- Danilenko V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State. 2004;46:595–599. doi: 10.1134/1.1711431. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

