The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia
- PMID: 35897781
- PMCID: PMC9330575
- DOI: 10.3390/ijms23158205
The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia
Abstract
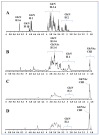
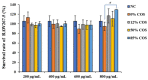
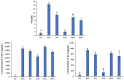
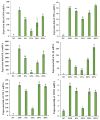
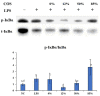
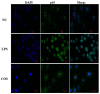
Endotoxemia is mainly caused by a massive burst of inflammatory cytokines as a result of lipopolysaccharide (LPS) invasion. Chitooligosaccharides (COS) is expected to be a potential drug for relieving endotoxemia due to its anti-inflammatory properties. However, the structural parameters of COS are often ambiguous, and the effect of degree of acetylation (DA) of COS on its anti-inflammatory remains unknown. In this study, four COSs with different DAs (0%, 12%, 50% and 85%) and the same oligomers distribution were successfully obtained. Their structures were confirmed by 1H NMR and MS analysis. Then, the effect of DA on the anti-inflammatory activity and relieving endotoxemia potential of COS was researched. The results revealed that COS with a DA of 12% had better anti-inflammatory activity than COSs with other DAs, mainly in inhibiting LPS-induced inflammatory cytokines burst, down-regulating its mRNA expression and reducing phosphorylation of IκBα. Furthermore, this COS showed an obviously protective effect on endotoxemia mice, such as inhibiting the increase in inflammatory cytokines and transaminases, alleviating the injury of liver and intestinal tissue. This study explored the effect of DA on the anti-inflammatory activity of COS for the first time and lays the foundation for the development of COS as an anti-inflammatory drug against endotoxemia.
Keywords: anti-inflammation; chitooligosaccharides; degree of acetylation; endotoxemia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

