Addiction of Cancer Stem Cells to MUC1-C in Triple-Negative Breast Cancer Progression
- PMID: 35897789
- PMCID: PMC9331006
- DOI: 10.3390/ijms23158219
Addiction of Cancer Stem Cells to MUC1-C in Triple-Negative Breast Cancer Progression
Abstract
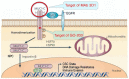
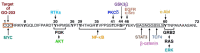
Triple-negative breast cancer (TNBC) is an aggressive malignancy with limited treatment options. TNBC progression is associated with expansion of cancer stem cells (CSCs). Few insights are available regarding druggable targets that drive the TNBC CSC state. This review summarizes the literature on TNBC CSCs and the compelling evidence that they are addicted to the MUC1-C transmembrane protein. In normal epithelia, MUC1-C is activated by loss of homeostasis and induces reversible wound-healing responses of inflammation and repair. However, in settings of chronic inflammation, MUC1-C promotes carcinogenesis. MUC1-C induces EMT, epigenetic reprogramming and chromatin remodeling in TNBC CSCs, which are dependent on MUC1-C for self-renewal and tumorigenicity. MUC1-C-induced lineage plasticity in TNBC CSCs confers DNA damage resistance and immune evasion by chronic activation of inflammatory pathways and global changes in chromatin architecture. Of therapeutic significance, an antibody generated against the MUC1-C extracellular domain has been advanced in a clinical trial of anti-MUC1-C CAR T cells and in IND-enabling studies for development as an antibody-drug conjugate (ADC). Agents targeting the MUC1-C cytoplasmic domain have also entered the clinic and are undergoing further development as candidates for advancing TNBC treatment. Eliminating TNBC CSCs will be necessary for curing this recalcitrant cancer and MUC1-C represents a promising druggable target for achieving that goal.
Keywords: CSC; DNA damage resistance; MUC1-C; TNBC; immune evasion.
Conflict of interest statement
N.Y. declares no potential conflicts of interest. D.K. has equity interests in Genus Oncology, Reata Pharmaceuticals and HillstreamBiopharma and is a paid consultant to Reata and CanBas.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

