RclS Sensor Kinase Modulates Virulence of Pseudomonas capeferrum
- PMID: 35897798
- PMCID: PMC9331949
- DOI: 10.3390/ijms23158232
RclS Sensor Kinase Modulates Virulence of Pseudomonas capeferrum
Abstract
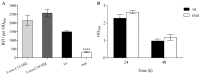
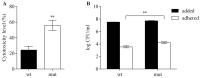
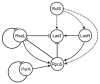
Signal transduction systems are the key players of bacterial adaptation and survival. The orthodox two-component signal transduction systems perceive diverse environmental stimuli and their regulatory response leads to cellular changes. Although rarely described, the unorthodox three-component systems are also implemented in the regulation of major bacterial behavior such as the virulence of clinically relevant pathogen P. aeruginosa. Previously, we described a novel three-component system in P. capeferrum WCS358 (RclSAR) where the sensor kinase RclS stimulates the intI1 transcription in stationary growth phase. In this study, using rclS knock-out mutant, we identified RclSAR regulon in P. capeferrum WCS358. The RNA sequencing revealed that activity of RclSAR signal transduction system is growth phase dependent with more pronounced regulatory potential in early stages of growth. Transcriptional analysis emphasized the role of RclSAR in global regulation and indicated the involvement of this system in regulation of diverse cellular activities such as RNA binding and metabolic and biocontrol processes. Importantly, phenotypic comparison of WCS358 wild type and ΔrclS mutant showed that RclS sensor kinase contributes to modulation of antibiotic resistance, production of AHLs and siderophore as well as host cell adherence and cytotoxicity. Finally, we proposed the improved model of interplay between RclSAR, RpoS and LasIR regulatory systems in P. capeferrum WCS358.
Keywords: Pseudomonas; RNA sequencing; antibiotic resistance; sensor kinase; three-component system; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


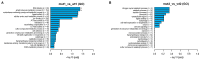

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

