Short-Term Effect of SARS-CoV-2 Spike Protein Receptor-Binding Domain-Specific Antibody Induction on Neutrophil-Mediated Immune Response in Mice
- PMID: 35897803
- PMCID: PMC9331224
- DOI: 10.3390/ijms23158234
Short-Term Effect of SARS-CoV-2 Spike Protein Receptor-Binding Domain-Specific Antibody Induction on Neutrophil-Mediated Immune Response in Mice
Abstract
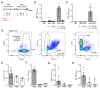
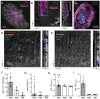
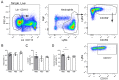
Vaccination protects against COVID-19 via the spike protein receptor-binding domain (RBD)-specific antibody formation, but it also affects the innate immunity. The effects of specific antibody induction on neutrophils that can cause severe respiratory inflammation are important, though not completely investigated. In the present study, using a mouse model mimicking SARS-CoV-2 virus particle inhalation, we investigated neutrophil phenotype and activity alterations in the presence of RBD-specific antibodies. Mice were immunized with RBD and a week after a strong antibody response establishment received 100 nm particles in the RBD solution. Control mice received injections of a phosphate buffer instead of RBD. We show that the application of 100 nm particles in the RBD solution elevates neutrophil recruitment to the blood and the airways of RBD-immunized mice rather than in control mice. Analysis of bone marrow cells of mice with induced RBD-specific antibodies revealed the increased population of CXCR2+CD101+ neutrophils. These neutrophils did not demonstrate an enhanced ability of neutrophil extracellular traps (NETs) formation compared to the neutrophils from control mice. Thus, the induction of RBD-specific antibodies stimulates the activation of mature neutrophils that react to RBD-coated particles without triggering excessive inflammation.
Keywords: SARS-CoV-2 infection; antibodies; mouse model; neutrophils; receptor-binding domain.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Föhse F.K., Geckin B., Overheul G.J., van de Maat J., Kilic G., Bulut O., Dijkstra H., Lemmers H., Sarlea S.A., Reijnders M., et al. The BNT162b2 MRNA Vaccine Against SARS-CoV-2 Reprograms Both Adaptive and Innate Immune Responses. medRxiv. 2021;03:21256520. doi: 10.2139/ssrn.3839624. - DOI
-
- Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. - DOI - PMC - PubMed
-
- Byazrova M.G., Kulemzin S.V., Astakhova E.A., Belovezhets T.N., Efimov G.A., Chikaev A.N., Kolotygin I.O., Gorchakov A.A., Taranin A.V., Filatov A.V. Memory B Cells Induced by Sputnik V Vaccination Produce SARS-CoV-2 Neutralizing Antibodies Upon Ex Vivo Restimulation. Front. Immunol. 2022;13:840707. doi: 10.3389/fimmu.2022.840707. - DOI - PMC - PubMed
-
- Kozlovskaya L.I., Piniaeva A.N., Ignatyev G.M., Gordeychuk I.V., Volok V.P., Rogova Y.V., Shishova A.A., Kovpak A.A., Ivin Y.Y., Antonova L.P., et al. Long-Term Humoral Immunogenicity, Safety and Protective Efficacy of Inactivated Vaccine against COVID-19 (CoviVac) in Preclinical Studies. Emerg. Microbes Infect. 2021;10:1790–1806. doi: 10.1080/22221751.2021.1971569. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

