Transcriptome Profile of Thyroid Glands in Bile Duct Ligation Mouse Model
- PMID: 35897811
- PMCID: PMC9332885
- DOI: 10.3390/ijms23158244
Transcriptome Profile of Thyroid Glands in Bile Duct Ligation Mouse Model
Abstract
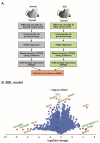
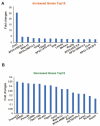
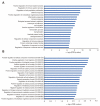
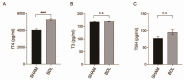
Thyroid hormone (TH) contributes to multiple cellular mechanisms in the liver, muscle cells, adipose tissue, and brain, etc. In particular, the liver is an important organ in TH metabolism for the conversion of thyronine (T4) into triiodothyronine (T3) by the deiodinase enzyme. TH levels were significantly decreased and thyroid-stimulating hormone (TSH) levels were significantly increased in patients with liver failure compared with normal subjects. Among liver failure diseases, hepatic encephalopathy (HE) deserves more attention because liver damage and neuropathologies occur simultaneously. Although there is numerous evidence of TH dysregulation in the HE model, specific mechanisms and genetic features of the thyroid glands in the HE model are not fully understood. Here, we investigated the significantly different genes in the thyroid glands of a bile duct ligation (BDL) mouse model as the HE model, compared to the thyroid glands of the control mouse using RNA sequencing. We also confirmed the alteration in mRNA levels of thyroid gland function-related genes in the BDL mouse model. Furthermore, we evaluated the increased level of free T4 and TSH in the BDL mouse blood. Thus, we emphasize the potential roles of TH in liver metabolism and suggest that thyroid dysfunction-related genes in the HE model should be highlighted for finding the appropriate solution for an impaired thyroid system in HE.
Keywords: RNA sequencing; bile duct ligation (BDL) model; hepatic encephalopathy (HE); thyroid gland; thyroid hormone (TH).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

