Hematopoietic-Mesenchymal Signals Regulate the Properties of Mesenchymal Stem Cells
- PMID: 35897814
- PMCID: PMC9330127
- DOI: 10.3390/ijms23158238
Hematopoietic-Mesenchymal Signals Regulate the Properties of Mesenchymal Stem Cells
Abstract
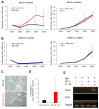
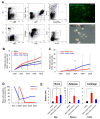
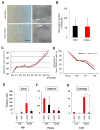
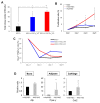
It is well known that the properties of hematopoietic stem/progenitor cells (HSCs), such as their self-renewal ability and multipotency, are maintained through interactions with mesenchymal stem/stromal cells (MSCs). MSCs are rare cells that are present in the bone marrow and are useful for clinical applications due to their functional ability. To obtain the necessary number of cells, MSCs must be cultured to expand, but this causes a remarkable decrease in stem cell properties, such as multipotency and proliferation ability. In this study, we show that the c-Mpl signal, which is related to the maintenance of hematopoietic stem cells, has an important effect on the proliferation and differentiation ability of MSCs. Utilizing a co-culture system comprising MSCs and HSCs, it is suggested that signaling from hematopoietic cells to MSCs supports cell proliferation. Interestingly, the enhanced proliferation ability of the HSCs was decreased in c-Mpl knock-out HSCs (c-Mpl-KO). In addition, the MSCs co-cultured with c-Mpl-KO HSCs had reduced MSC marker expression (PDGFRa and Sca-1) compared to the MSCs co-cultured with c-Mpl-wild-type HSCs. These results suggest that a hematopoietic-mesenchymal signal exists, and that the state of the HSCs is important for the stability of MSC properties.
Keywords: hematopoietic stem cells; hematopoietic–mesenchymal cell interaction; hematopoietic–mesenchymal signaling; mesenchymal stem cells.
Conflict of interest statement
Atsuhiko Hikita is affiliated with and is the endowed chair of FUJISOFT INCORPORATED (until 20 October 2020) and the CPC Corporation, KYOWA Co., Ltd., Kanto Chemical Co Inc., and the Nichirei Corporation (from 1 July 2021). All other authors have no conflict of interest.
Figures


References
-
- Uejima S., Okada K., Kagami H., Taguchi A., Ueda M. Bone marrow stromal cell therapy improves femoral bone mineral density and mechanical strength in ovariectomized rats. Cytotherapy. 2008;10:479–489. - PubMed
-
- Petrenko Y., Vackova I., Kekulova K., Chudickova M., Koci Z., Turnovcova K., Kupcova Skalnikova H., Vodicka P., Kubinova S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020;10:4290. - PMC - PubMed
-
- Consentius C., Mirenska A., Jurisch A., Reinke S., Scharm M., Zenclussen A.C., Hennig C., Volk H.D. In situ detection of CD73+ CD90+ CD105+ lineage: Mesenchymal stromal cells in human placenta and bone marrow specimens by chipcytometry. Cytom. A. 2018;93:889–893. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

