Myeloperoxidase as a Marker to Differentiate Mouse Monocyte/Macrophage Subsets
- PMID: 35897821
- PMCID: PMC9330004
- DOI: 10.3390/ijms23158246
Myeloperoxidase as a Marker to Differentiate Mouse Monocyte/Macrophage Subsets
Abstract
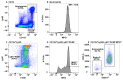
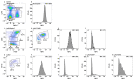
Macrophages are present in every tissue in the body and play essential roles in homeostasis and host defense against microorganisms. Some tissue macrophages derive from the yolk sac/fetal liver that populate tissues for life. Other tissue macrophages derive from monocytes that differentiate in the bone marrow and circulate through tissues via the blood and lymphatics. Circulating monocytes are very plastic and differentiate into macrophages with specialized functions upon entering tissues. Specialized monocyte/macrophage subsets have been difficult to differentiate based on cell surface markers. Here, using a combination of "pan" monocyte/macrophage markers and flow cytometry, we asked whether myeloperoxidase (MPO) could be used as a marker of pro-inflammatory monocyte/macrophage subsets. MPO is of interest because of its potent microbicidal activity. In wild-type SPF housed mice, we found that MPO+ monocytes/macrophages were present in peripheral blood, spleen, small and large intestines, and mesenteric lymph nodes, but not the central nervous system. Only monocytes/macrophages that expressed cell surface F4/80 and/or Ly6C co-expressed MPO with the highest expression in F4/80HiLy6CHi subsets regardless of tissue. These cumulative data indicate that MPO expression can be used as an additional marker to differentiate between monocyte/macrophage subsets with pro-inflammatory and microbicidal activity in a variety of tissues.
Keywords: macrophage; monocyte; myeloperoxidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

