Sogatella furcifera Saliva Mucin-like Protein Is Required for Feeding and Induces Rice Defences
- PMID: 35897828
- PMCID: PMC9332473
- DOI: 10.3390/ijms23158239
Sogatella furcifera Saliva Mucin-like Protein Is Required for Feeding and Induces Rice Defences
Abstract
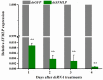
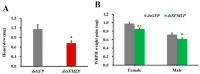
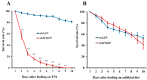
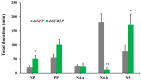
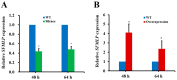
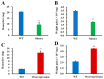
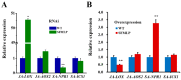
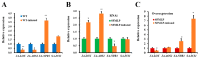
The white-backed planthopper (WBPH), Sogatella furcifera, is one of the most important piercing-sucking pests of rice (Oryza sativa) in Asia. Mucin-like salivary protein (SFMLP) is highly expressed in the salivary glands of WBPH, which plays an important role in WBPH feeding. In this study, WBPH injected with dsSFMLP had difficulty in sucking phloem sap from rice plants, which significantly reduced their food intake, weight, and survival. In contrast, the knockdown of the SFMLP gene had only a marginal effect on the survival of WBPH fed an artificial diet. Further studies showed that silencing SFMLP resulted in the short and single-branched salivary sheaths secretion and less formation of salivary flanges in rice. These data suggest that SFMLP is involved in the formation of the salivary sheath and is essential for feeding in WBPH. Overexpression of the SFMLP gene in rice plants promoted the feeding of WBPH, whereas silencing the gene in rice plants significantly decreased WBPH performance. Additionally, it was found that overexpression of SFMLP in rice plants elicited the signalling pathway of SA (salicylic acid) while suppressing JA (jasmonic acid); in contrast, silencing of the SFMLP gene in rice plants showed the opposite results. This study clarified the function of SFMLP in WBPH feeding as well as mediating rice defences.
Keywords: Sogatella furcifera; mucin-like protein; plant-insect interaction; rice defense response; salivary proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Felton G.W., Chung S.H., Hernandez M.G.E., Louis J., Peiffer M., Tian D. Herbivore oral secretions are the first line of protection against plant-induced defences. Annu. Plant Rev. 2014;47:37–76.
-
- Miles P.W. Aphid saliva. Biol. Rev. Camb. Philos. Soc. 1999;74:41–85. doi: 10.1017/S0006323198005271. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

