COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR
- PMID: 35897843
- PMCID: PMC9332821
- DOI: 10.3390/ijms23158267
COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR
Abstract
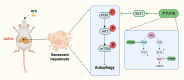
We previously found that the disorder of soluble epoxide hydrolase (sEH)/cyclooxygenase-2 (COX-2)-mediated arachidonic acid (ARA) metabolism contributes to the pathogenesis of the non-alcoholic fatty liver disease (NAFLD) in mice. However, the exact mechanism has not been elucidated. Accumulating evidence points to the essential role of cellular senescence in NAFLD. Herein, we investigated whether restoring the balance of sEH/COX-2-mediated ARA metabolism attenuated NAFLD via hepatocyte senescence. A promised dual inhibitor of sEH and COX-2, PTUPB, was used in our study to restore the balance of sEH/COX-2-mediated ARA metabolism. In vivo, NAFLD was induced by a high-fat diet (HFD) using C57BL/6J mice. In vitro, mouse hepatocytes (AML12) and mouse hepatic astrocytes (JS1) were used to investigate the effects of PTUPB on palmitic acid (PA)-induced hepatocyte senescence and its mechanism. PTUPB alleviated liver injury, decreased collagen and lipid accumulation, restored glucose tolerance, and reduced hepatic triglyceride levels in HFD-induced NAFLD mice. Importantly, PTUPB significantly reduced the expression of liver senescence-related molecules p16, p53, and p21 in HFD mice. In vitro, the protein levels of γH2AX, p53, p21, COX-2, and sEH were increased in AML12 hepatocytes treated with PA, while Ki67 and PCNA were significantly decreased. PTUPB decreased the lipid content, the number of β-gal positive cells, and the expression of p53, p21, and γH2AX proteins in AML12 cells. Meanwhile, PTUPB reduced the activation of hepatic astrocytes JS1 by slowing the senescence of AML12 cells in a co-culture system. It was further observed that PTUPB enhanced the ratio of autophagy-related protein LC3II/I in AML12 cells, up-regulated the expression of Fundc1 protein, reduced p62 protein, and suppressed hepatocyte senescence. In addition, PTUPB enhanced hepatocyte autophagy by inhibiting the PI3K/AKT/mTOR pathway through Sirt1, contributing to the suppression of senescence. PTUPB inhibits the PI3K/AKT/mTOR pathway through Sirt1, improves autophagy, slows down the senescence of hepatocytes, and alleviates NAFLD.
Keywords: COX-2/sEH dual inhibitor; autophagy; non-alcoholic fatty liver disease; senescence.
Conflict of interest statement
The authors declared no conflict of interests.
Figures
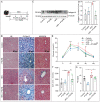


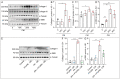

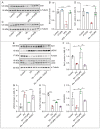
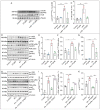
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

