Recent Applications of the Multicomponent Synthesis for Bioactive Pyrazole Derivatives
- PMID: 35897899
- PMCID: PMC9331265
- DOI: 10.3390/molecules27154723
Recent Applications of the Multicomponent Synthesis for Bioactive Pyrazole Derivatives
Abstract
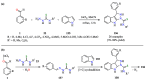
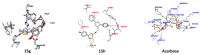
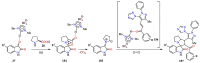
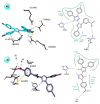
Pyrazole and its derivatives are considered a privileged N-heterocycle with immense therapeutic potential. Over the last few decades, the pot, atom, and step economy (PASE) synthesis of pyrazole derivatives by multicomponent reactions (MCRs) has gained increasing popularity in pharmaceutical and medicinal chemistry. The present review summarizes the recent developments of multicomponent reactions for the synthesis of biologically active molecules containing the pyrazole moiety. Particularly, it covers the articles published from 2015 to date related to antibacterial, anticancer, antifungal, antioxidant, α-glucosidase and α-amylase inhibitory, anti-inflammatory, antimycobacterial, antimalarial, and miscellaneous activities of pyrazole derivatives obtained exclusively via an MCR. The reported analytical and activity data, plausible synthetic mechanisms, and molecular docking simulations are organized in concise tables, schemes, and figures to facilitate comparison and underscore the key points of this review. We hope that this review will be helpful in the quest for developing more biologically active molecules and marketed drugs containing the pyrazole moiety.
Keywords: biological activity; drug discovery; medicinal chemistry; multicomponent reactions (MCRs); pyrazole derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












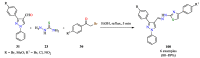






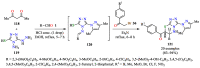

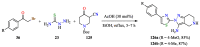


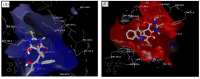














References
-
- Hulme C. Applications of multicomponent reactions in drug discovery–lead generation to process development. In: Zhu J., Bienaymé H., editors. Multicomponent Reactions. 1st ed. Volume 1. WILEY-VCH; Weinheim, German: 2005. pp. 311–341.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

