Halogenated Flavonoid Derivatives Display Antiangiogenic Activity
- PMID: 35897938
- PMCID: PMC9331694
- DOI: 10.3390/molecules27154757
Halogenated Flavonoid Derivatives Display Antiangiogenic Activity
Abstract
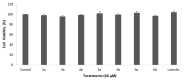
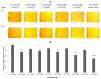
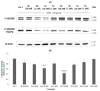
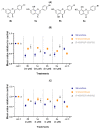
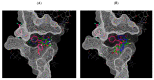
Antiangiogenic agents attenuate tumours' growth and metastases and are therefore beneficial as an adjuvant or standalone cancer regimen. Drugs with dual antiproliferative and antiangiogenic activities can achieve anticancer efficacy and overcome acquired resistance. In this study, synthetic flavones (5a,b) with reported anticancer activity, and derivatives (4b and 6a), exhibited significant inhibition of endothelial cell tube formation (40-55%, 12 h) at 1 µM, which is comparable to sunitinib (50% inhibition at 1 µM, 48 h). Flavones (4b, 5a,b and 6a) also showed 25-37% reduction in HUVECs migration at 10 µM. In a Western blotting assay, 5a and 5b subdued VEGFR2 phosphorylation by 37% and 57%, respectively, suggesting that VEGFR2 may be their main antiangiogenic target. 5b displayed the best docking fit with VEGFR2 in an in silico study, followed by 5a, emphasizing the importance of the 7-hydroxyl group accompanied by a 4-C=S for activity. Conversely, derivatives with a 4-carbonyl moiety fitted poorly into the target's binding pocket, suggesting that their antiangiogenic activity depends on a different target. This study provides valuable insight into the Structure Activity Relationships (SAR) and modes of action of halogenated flavones with VEGFR2 and highlights their therapeutic potential as antiangiogenic/anticancer lead compounds.
Keywords: SAR; angiogenesis; antiangiogenic; cancer; flavones; flavonoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

