Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells
- PMID: 35898110
- PMCID: PMC9336479
- DOI: 10.1080/19490976.2022.2096994
Phospholipid metabolites of the gut microbiota promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells
Abstract
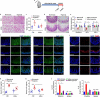
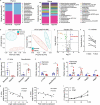
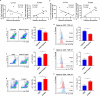
Gastrointestinal dysfunction is a common symptom of acute mountain sickness (AMS). The gut microbiota and γδ T cells play critical roles in intestinal disease. However, the mechanistic link between the microbiota and γδ T cells in hypoxia-induced intestinal injury remains unclear. Here, we show that hypoxia-induced intestinal damage was significantly alleviated after microbiota depletion with antibiotics. Hypoxia modulated gut microbiota composition by promoting antimicrobial peptides angiogenin-4 secretions. The abundance of Clostridium in the gut of mice after hypoxia significantly decreased, while the abundance of Desulfovibrio significantly increased. Furthermore, Desulfovibrio-derived phosphatidylethanolamine and phosphatidylcholine promoted γδ T cell activation. In CD1d-deficient mice, the levels of intraepithelial IL-17A and γδ T cells and intestinal damage were significantly decreased compared with those in wild-type mice under hypoxia. Mechanistically, phospholipid metabolites from Desulfovibrio are presented by intestinal epithelial CD1d to induce the proliferation of IL-17A-producing γδ T cells, which aggravates intestinal injury. Gut microbiota-derived metabolites promote hypoxia-induced intestinal injury via CD1d-dependent γδ T cells, suggesting that phospholipid metabolites and γδ T cells can be targets for AMS therapy.
Keywords: Gut microbiota; hypoxia; intestinal injury; metabolites; γδ T cells.
Conflict of interest statement
The authors report no conflict of interest.
Figures



Similar articles
-
Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells.Cell Rep. 2021 Jul 6;36(1):109332. doi: 10.1016/j.celrep.2021.109332. Cell Rep. 2021. PMID: 34233192
-
Infiltrated IL-17A-producing gamma delta T cells play a protective role in sepsis-induced liver injury and are regulated by CCR6 and gut commensal microbes.Front Cell Infect Microbiol. 2023 Jul 5;13:1149506. doi: 10.3389/fcimb.2023.1149506. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37475963 Free PMC article.
-
Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis.Hepatology. 2016 Aug;64(2):616-31. doi: 10.1002/hep.28644. Epub 2016 Jun 29. Hepatology. 2016. PMID: 27178735
-
New Aspect of Liver IL-17+γδ T Cells.Mol Immunol. 2019 Mar;107:41-43. doi: 10.1016/j.molimm.2018.12.030. Epub 2019 Jan 12. Mol Immunol. 2019. PMID: 30641412 Review.
-
Crosstalk between γδ T cells and the microbiota.Nat Microbiol. 2021 Sep;6(9):1110-1117. doi: 10.1038/s41564-021-00948-2. Epub 2021 Aug 2. Nat Microbiol. 2021. PMID: 34341528 Review.
Cited by
-
Tryptophan Attenuates Chronic Restraint Stress-Induced Intestinal Injury Through Modulation of Intestinal Barrier Integrity and Gut Microbiota Homeostasis.Nutrients. 2025 Mar 11;17(6):975. doi: 10.3390/nu17060975. Nutrients. 2025. PMID: 40290020 Free PMC article.
-
γδ T cells: origin and fate, subsets, diseases and immunotherapy.Signal Transduct Target Ther. 2023 Nov 22;8(1):434. doi: 10.1038/s41392-023-01653-8. Signal Transduct Target Ther. 2023. PMID: 37989744 Free PMC article. Review.
-
Faecalibacterium duncaniae Mitigates Intestinal Barrier Damage in Mice Induced by High-Altitude Exposure by Increasing Levels of 2-Ketoglutaric Acid.Nutrients. 2025 Apr 19;17(8):1380. doi: 10.3390/nu17081380. Nutrients. 2025. PMID: 40284246 Free PMC article.
-
High-altitude-induced alterations in intestinal microbiota.Front Microbiol. 2024 May 9;15:1369627. doi: 10.3389/fmicb.2024.1369627. eCollection 2024. Front Microbiol. 2024. PMID: 38784803 Free PMC article. Review.
-
Immunogenic molecules associated with gut bacterial cell walls: chemical structures, immune-modulating functions, and mechanisms.Protein Cell. 2023 Oct 25;14(10):776-785. doi: 10.1093/procel/pwad016. Protein Cell. 2023. PMID: 37013853 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
