Comparative Plastid Genomics of Green-Colored Dinoflagellates Unveils Parallel Genome Compaction and RNA Editing
- PMID: 35898209
- PMCID: PMC9309888
- DOI: 10.3389/fpls.2022.918543
Comparative Plastid Genomics of Green-Colored Dinoflagellates Unveils Parallel Genome Compaction and RNA Editing
Abstract
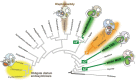
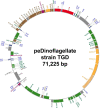
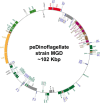
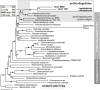
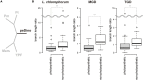
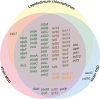
Dinoflagellates possess plastids that are diverse in both pigmentation and evolutionary background. One of the plastid types found in dinoflagellates is pigmented with chlorophylls a and b (Chl a + b) and originated from the endosymbionts belonging to a small group of green algae, Pedinophyceae. The Chl a + b-containing plastids have been found in three distantly related dinoflagellates Lepidodinium spp., strain MGD, and strain TGD, and were proposed to be derived from separate partnerships between a dinoflagellate (host) and a pedinophycean green alga (endosymbiont). Prior to this study, a plastid genome sequence was only available for L. chlorophorum, which was reported to bear the features that were not found in that of the pedinophycean green alga Pedinomonas minor, a putative close relative of the endosymbiont that gave rise to the current Chl a + b-containing plastid. In this study, we sequenced the plastid genomes of strains MGD and TGD to compare with those of L. chlorophorum as well as pedinophycean green algae. The mapping of the RNA-seq reads on the corresponding plastid genome identified RNA editing on plastid gene transcripts in the three dinoflagellates. Further, the comparative plastid genomics revealed that the plastid genomes of the three dinoflagellates achieved several features, which are not found in or much less obvious than the pedinophycean plastid genomes determined to date, in parallel.
Keywords: Lepidodinium; Pedinophyceae; RNA editing; complex plastids; peDinoflagellates; plastid replacements; serial secondary endosymbiosis.
Copyright © 2022 Matsuo, Morita, Nakayama, Yazaki, Sarai, Takahashi, Iwataki and Inagaki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Plastid genome-based phylogeny pinpointed the origin of the green-colored plastid in the dinoflagellate Lepidodinium chlorophorum.Genome Biol Evol. 2015 Apr 2;7(4):1133-40. doi: 10.1093/gbe/evv060. Genome Biol Evol. 2015. PMID: 25840416 Free PMC article.
-
Patterns in evolutionary origins of heme, chlorophyll a and isopentenyl diphosphate biosynthetic pathways suggest non-photosynthetic periods prior to plastid replacements in dinoflagellates.PeerJ. 2018 Aug 3;6:e5345. doi: 10.7717/peerj.5345. eCollection 2018. PeerJ. 2018. PMID: 30083465 Free PMC article.
-
A phylogenetic mosaic plastid proteome and unusual plastid-targeting signals in the green-colored dinoflagellate Lepidodinium chlorophorum.BMC Evol Biol. 2010 Jun 21;10:191. doi: 10.1186/1471-2148-10-191. BMC Evol Biol. 2010. PMID: 20565933 Free PMC article.
-
Interpreting the complexities of the plastid genome in dinoflagellates: a mini-review of recent advances.Plant Mol Biol. 2024 Oct 21;114(6):114. doi: 10.1007/s11103-024-01511-3. Plant Mol Biol. 2024. PMID: 39432142 Review.
-
A new scenario of plastid evolution: plastid primary endosymbiosis before the divergence of the "Plantae," emended.J Plant Res. 2005 Aug;118(4):247-55. doi: 10.1007/s10265-005-0219-1. Epub 2005 Jul 20. J Plant Res. 2005. PMID: 16032387 Review.
Cited by
-
A decade of dinoflagellate genomics illuminating an enigmatic eukaryote cell.BMC Genomics. 2024 Oct 4;25(1):932. doi: 10.1186/s12864-024-10847-5. BMC Genomics. 2024. PMID: 39367346 Free PMC article. Review.
-
Modes and mechanisms for the inheritance of mitochondria and plastids in pathogenic protists.PLoS Pathog. 2025 Jan 23;21(1):e1012835. doi: 10.1371/journal.ppat.1012835. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39847585 Free PMC article. Review.
-
New plastids, old proteins: repeated endosymbiotic acquisitions in kareniacean dinoflagellates.EMBO Rep. 2024 Apr;25(4):1859-1885. doi: 10.1038/s44319-024-00103-y. Epub 2024 Mar 18. EMBO Rep. 2024. PMID: 38499810 Free PMC article.
-
Encyclopedia of Family A DNA Polymerases Localized in Organelles: Evolutionary Contribution of Bacteria Including the Proto-Mitochondrion.Mol Biol Evol. 2024 Feb 1;41(2):msae014. doi: 10.1093/molbev/msae014. Mol Biol Evol. 2024. PMID: 38271287 Free PMC article.
-
Complete Mitochondrial Genomes of Ancyromonads Provide Clues for the Gene Content and Genome Structures of Ancestral Mitochondria.J Eukaryot Microbiol. 2025 May-Jun;72(3):e70012. doi: 10.1111/jeu.70012. J Eukaryot Microbiol. 2025. PMID: 40384044 Free PMC article.
References
-
- Barbrook A. C., Howe C. J., Nisbet R. E. R. (2019). Breaking up is hard to do: the complexity of the dinoflagellate chloroplast genome. Perspect. Phycol. 6 31–37. 10.1127/pip/2018/0084 - DOI
-
- Bjørnland T., Haxo F. T., Liaaen-Jensen S. (2003). Carotenoids of the Florida red tide dinoflagellate Karenia brevis. Biochem. Syst. Ecol. 31 1147–1162. 10.1016/S0305-1978(03)00044-9 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

