Long-Term Mild Heat Causes Post-Mitotic Pollen Abortion Through a Local Effect on Flowers
- PMID: 35898227
- PMCID: PMC9310381
- DOI: 10.3389/fpls.2022.925754
Long-Term Mild Heat Causes Post-Mitotic Pollen Abortion Through a Local Effect on Flowers
Abstract
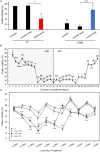
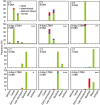
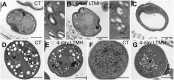
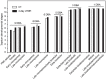
Crop reproductive success is significantly challenged by heatwaves, which are increasing in frequency and severity globally. Heat-induced male sterility is mainly due to aborted pollen development, but it is not clear whether this is through direct or systemic effects. Here, long-term mild heat (LTMH) treatment, mimicking a heatwave, was applied locally to tomato flowers or whole plants and followed up by cytological, transcriptomic, and biochemical analyses. By analyzing pollen viability, LTMH was shown to act directly on the flowers and not via effects on other plant tissue. The meiosis to early microspore stage of pollen development was the most sensitive to LTMH and 3 days of exposure around this period was sufficient to significantly reduce pollen viability at the flower anthesis stage. Extensive cytological analysis showed that abnormalities in pollen development could first be observed after pollen mitosis I, while no deviations in tapetum development were observed. Transcriptomic and biochemical analyses suggested that pollen development suffered from tapetal ER stress and that there was a limited role for oxidative stress. Our results provide the first evidence that heat acts directly on flowers to induce pollen sterility, and that the molecular-physiological responses of developing anthers to the LTMH are different from those to severe heat shock.
Keywords: long-term mild heat; oxidative stress; pollen development; tapetum development; tomato (Solanum lycopersicum).
Copyright © 2022 Xu, Jansma, Wolters-Arts, de Groot, Jansen and Rieu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Low Salicylic Acid Level Improves Pollen Development Under Long-Term Mild Heat Conditions in Tomato.Front Plant Sci. 2022 Apr 11;13:828743. doi: 10.3389/fpls.2022.828743. eCollection 2022. Front Plant Sci. 2022. PMID: 35481151 Free PMC article.
-
[Cytological observation of anther abortion and starch distribution of a cytoplasm male sterile pepper (Capsicum annum L.)].Fen Zi Xi Bao Sheng Wu Xue Bao. 2008 Aug;41(4):283-93. Fen Zi Xi Bao Sheng Wu Xue Bao. 2008. PMID: 18959002 Chinese.
-
The tapetal tissue is essential for the maintenance of redox homeostasis during microgametogenesis in tomato.Plant J. 2022 Dec;112(5):1281-1297. doi: 10.1111/tpj.16014. Epub 2022 Nov 19. Plant J. 2022. PMID: 36307971 Free PMC article.
-
Thermal stress impacts reproductive development and grain yield in rice.Plant Physiol Biochem. 2017 Jun;115:57-72. doi: 10.1016/j.plaphy.2017.03.011. Epub 2017 Mar 16. Plant Physiol Biochem. 2017. PMID: 28324683 Review.
-
Heat stress regimes for the investigation of pollen thermotolerance in crop plants.Plant Reprod. 2016 Jun;29(1-2):93-105. doi: 10.1007/s00497-016-0281-y. Epub 2016 Mar 25. Plant Reprod. 2016. PMID: 27016360 Review.
Cited by
-
Asynapsis and meiotic restitution in tomato male meiosis induced by heat stress.Front Plant Sci. 2023 Jul 13;14:1210092. doi: 10.3389/fpls.2023.1210092. eCollection 2023. Front Plant Sci. 2023. PMID: 37521921 Free PMC article.
-
γ-Aminobutyric Acid Alleviates Programmed Cell Death in Two Brassica Species Under Cadmium Stress.Int J Mol Sci. 2024 Dec 27;26(1):129. doi: 10.3390/ijms26010129. Int J Mol Sci. 2024. PMID: 39795987 Free PMC article.
-
Characterization and Early Response of the DEAD Gene Family to Heat Stress in Tomato.Plants (Basel). 2025 Apr 9;14(8):1172. doi: 10.3390/plants14081172. Plants (Basel). 2025. PMID: 40284060 Free PMC article.
References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

