Cations Do Not Alter the Membrane Structure of POPC-A Lipid With an Intermediate Area
- PMID: 35898308
- PMCID: PMC9312375
- DOI: 10.3389/fmolb.2022.926591
Cations Do Not Alter the Membrane Structure of POPC-A Lipid With an Intermediate Area
Abstract
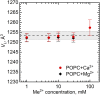
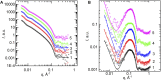
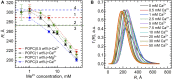
Combining small-angle neutron scattering (SANS), small-angle X-ray scattering (SAXS), and densitometric measurements, we have studied the interactions of the divalent cations Ca2+ and Mg2+ with the lipid vesicles prepared of a mixed-chain palmitoyl-oleoyl-phosphatidylcholine (POPC) at 25°C. The structural parameters of the POPC bilayer, such as the bilayer thickness, lateral area, and volume per lipid, displayed no changes upon the ion addition at concentrations up to 30 mM and minor changes at > 30 mM Ca2+ and Mg2+, while some decrease in the vesicle radius was observed over the entire concentration range studied. This examination allows us to validate the concept of lipid-ion interactions governed by the area per lipid suggested previously and to propose the mixed mode of those interactions that emerge in the POPC vesicles. We speculate that the average area per POPC lipid that corresponds to the cutoff length of lipid-ion interactions generates an equal but opposite impact on ion bridges and separate lipid-ion pairs. As a result of the dynamic equilibrium, the overall structural properties of bilayers are not affected. As the molecular mechanism proposed is affected by the structural properties of a particular lipid, it might help us to understand the fundamentals of processes occurring in complex multicomponent membrane systems.
Keywords: SANS; SAXS; cations; densitometry; lipid bilayer; structure.
Copyright © 2022 Kurakin, Ivankov, Skoi, Kuklin, Uhríková and Kučerka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

