Caenorhabditis nematodes colonize ephemeral resource patches in neotropical forests
- PMID: 35898425
- PMCID: PMC9309040
- DOI: 10.1002/ece3.9124
Caenorhabditis nematodes colonize ephemeral resource patches in neotropical forests
Erratum in
-
Erratum: Corrigendum.Ecol Evol. 2022 Sep 14;12(9):e9317. doi: 10.1002/ece3.9317. eCollection 2022 Sep. Ecol Evol. 2022. PMID: 36177143 Free PMC article.
Abstract
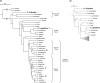
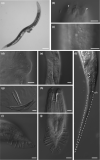
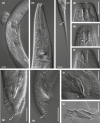
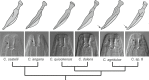
Factors shaping the distribution and abundance of species include life-history traits, population structure, and stochastic colonization-extinction dynamics. Field studies of model species groups help reveal the roles of these factors. Species of Caenorhabditis nematodes are highly divergent at the sequence level but exhibit highly conserved morphology, and many of these species live in sympatry on microbe-rich patches of rotten material. Here, we use field experiments and large-scale opportunistic collections to investigate species composition, abundance, and colonization efficiency of Caenorhabditis species in two of the world's best-studied lowland tropical field sites: Barro Colorado Island in Panamá and La Selva in Sarapiquí, Costa Rica. We observed seven species of Caenorhabditis, four of them known only from these collections. We formally describe two species and place them within the Caenorhabditis phylogeny. While these localities contain species from many parts of the phylogeny, both localities were dominated by globally distributed androdiecious species. We found that Caenorhabditis individuals were able to colonize baits accessible only through phoresy and preferentially colonized baits that were in direct contact with the ground. We estimate the number of colonization events per patch to be low.
Keywords: Caenorhabditis; dispersal; nematode; population biology; species description.
© 2022 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
None to declare.
Figures





References
-
- Alphonse, E. S. (1956). Guaymi; Grammar and Dictionary, with Some Ethnological Notes. Bureau of American Ethnology Bulletin, 162, 1–128.
-
- Andrews, S . (2010). FastQC: A quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
-
- Baermann, G. (1917). Ein Einfache Methode Zur Auffindung von Anklyostomum (Nematoden) Larven in Erdproben. Geneesk. Tijdschr. Nederlandsch‐Indie, 67, 131–137.
-
- Baldwin, J. G. , Frisse, L. M. , Vida, J. T. , Eddleman, C. D. , & Thomas, W. K. (1997). An evolutionary framework for the study of developmental evolution in a set of nematodes related to Caenorhabditis elegans . Molecular Phylogenetics and Evolution, 8(2), 249–259. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

