Homeostasis Maintenance in Plasmodium-Infected Placentas: Is There a Role for Placental Autophagy During Malaria in Pregnancy?
- PMID: 35898514
- PMCID: PMC9309427
- DOI: 10.3389/fimmu.2022.931034
Homeostasis Maintenance in Plasmodium-Infected Placentas: Is There a Role for Placental Autophagy During Malaria in Pregnancy?
Abstract
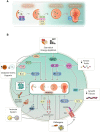
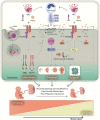
Malaria represents a significant public health burden to populations living in developing countries. The disease takes a relevant toll on pregnant women, who are more prone to developing severe clinical manifestations. Inflammation triggered in response to P. falciparum sequestration inside the placenta leads to physiological and structural changes in the organ, reflecting locally disrupted homeostasis. Altogether, these events have been associated with poor gestational outcomes, such as intrauterine growth restriction and premature delivery, contributing to the parturition of thousands of African children with low birth weight. Despite significant advances in the field, the molecular mechanisms that govern these outcomes are still poorly understood. Herein, we discuss the idea of how some housekeeping molecular mechanisms, such as those related to autophagy, might be intertwined with the outcomes of malaria in pregnancy. We contextualize previous findings suggesting that placental autophagy is dysregulated in P. falciparum-infected pregnant women with complementary research describing the importance of autophagy in healthy pregnancies. Since the functional role of autophagy in pregnancy outcomes is still unclear, we hypothesize that autophagy might be essential for circumventing inflammation-induced stress in the placenta, acting as a cytoprotective mechanism that attempts to ensure local homeostasis and better gestational prognosis in women with malaria in pregnancy.
Keywords: Autophagy; Inflammation; Malaria; Placenta; Plasmodium; Pregnancy.
Copyright © 2022 Barateiro, Junior, Epiphanio and Marinho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . World Malaria Report 2021 Geneva: World Health Organization; (2021). 322 p. Licence: CC BY-NC-SA 3.0 IGO.
-
- World Health Organization . World Malaria Report 2019. Geneva: World Health Organization; (2019). 232 p. Licence: CC BY-NC-SA 3.0 IGO.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

