Protective Efficacy of H9N2 Avian Influenza Vaccines Inactivated by Ionizing Radiation Methods Administered by the Parenteral or Mucosal Routes
- PMID: 35898545
- PMCID: PMC9309530
- DOI: 10.3389/fvets.2022.916108
Protective Efficacy of H9N2 Avian Influenza Vaccines Inactivated by Ionizing Radiation Methods Administered by the Parenteral or Mucosal Routes
Abstract
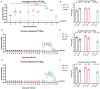
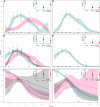
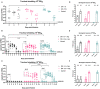
H9N2 viruses have become, over the last 20 years, one of the most diffused poultry pathogens and have reached a level of endemicity in several countries. Attempts to control the spread and reduce the circulation of H9N2 have relied mainly on vaccination in endemic countries. However, the high level of adaptation to poultry, testified by low minimum infectious doses, replication to high titers, and high transmissibility, has severely hampered the results of vaccination campaigns. Commercially available vaccines have demonstrated high efficacy in protecting against clinical disease, but variable results have also been observed in reducing the level of replication and viral shedding in domestic poultry species. Antigenic drift and increased chances of zoonotic infections are the results of incomplete protection offered by the currently available vaccines, of which the vast majority are based on formalin-inactivated whole virus antigens. In our work, we evaluated experimental vaccines based on an H9N2 virus, inactivated by irradiation treatment, in reducing viral shedding upon different challenge doses and compared their efficacy with formalin-inactivated vaccines. Moreover, we evaluated mucosal delivery of inactivated antigens as an alternative route to subcutaneous and intramuscular vaccination. The results showed complete protection and prevention of replication in subcutaneously vaccinated Specific Pathogen Free White Leghorn chickens at low-to-intermediate challenge doses but a limited reduction of shedding at a high challenge dose. Mucosally vaccinated chickens showed a more variable response to experimental infection at all tested challenge doses and the main effect of vaccination attained the reduction of infected birds in the early phase of infection. Concerning mucosal vaccination, the irradiated vaccine was the only one affording complete protection from infection at the lowest challenge dose. Vaccine formulations based on H9N2 inactivated by irradiation demonstrated a potential for better performances than vaccines based on the formalin-inactivated antigen in terms of reduction of shedding and prevention of infection.
Keywords: H9N2; formalin-inactivated; irradiated; mucosal; subcutaneous; vaccines.
Copyright © 2022 Bortolami, Mazzetto, Kangethe, Wijewardana, Barbato, Porfiri, Maniero, Mazzacan, Budai, Marciano, Panzarin, Terregino, Bonfante and Cattoli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

