Chimeric RNA TNNI2-ACTA1-V1 Regulates Cell Proliferation by Regulating the Expression of NCOA3
- PMID: 35898549
- PMCID: PMC9309209
- DOI: 10.3389/fvets.2022.895190
Chimeric RNA TNNI2-ACTA1-V1 Regulates Cell Proliferation by Regulating the Expression of NCOA3
Abstract
Chimeric RNA is a crucial target for tumor diagnosis and drug therapy, also having its unique biological role in normal tissues. TNNI2-ACTA1-V1 (TA-V1), a chimeric RNA discovered by our laboratory in porcine muscle tissue, can inhibit the proliferation of Porcine Skeletal Muscle Satellite Cells (PSCs). The regulatory mechanism of TA-V1 in PSCs remains unclear, but we speculate that NCOA3, DDR2 and RDX may be the target genes of TA-V1. In this study, we explored the effects of NCOA3, DDR2 and RDX on cell viability and cell proliferation by CCK-8 assay, EdU staining and flow cytometry. Furthermore, the regulatory pathway of proliferation in PSCs mediated by TA-V1 through NCOA3 or CyclinD1 was elucidated by co-transfection and co-immunoprecipitation (Co-IP). The results revealed that overexpression of NCOA3 significantly increased cell viability and the expression level of CyclinD1, and also promotes cell proliferation by changing cells from the G1 phase to the S phase. In addition, inhibiting the expression of NCOA3 substantially reduced cell viability and inhibited cell proliferation. Overexpression of DDR2 and RDX had no significant effect on cell viability and proliferation. Co-transfection experiments showed that NCOA3 could rescue the proliferation inhibition of PSCs caused by TA-V1. Co-IP assay indicated that TA-V1 directly interacts with NCOA3. Our study explores the hypothesis that TA-V1 directly regulates NCOA3, indirectly regulating CyclinD1, thereby regulating PSCs proliferation. We provide new putative mechanisms of porcine skeletal muscle growth and lay the foundation for the study of chimeric RNA in normal tissues.
Keywords: NCOA3; cell proliferation; chimeric RNA TA-V1; growth; porcine skeletal muscle.
Copyright © 2022 Liu, Li, Hao, Lin, Xia, Zhu, Yang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










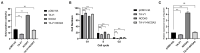

References
-
- Yuan H, Qin F, Movassagh M, Park H, Golden W, Xie Z, et al. A chimeric RNA characteristic of rhabdomyosarcoma in normal myogenesis process. Cancer Disc. (2013) 3:1394–403. 10.1158/2159-8290.CD-13-0186 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

