Defining a role for Interferon Epsilon in normal and complicated pregnancies
- PMID: 35898609
- PMCID: PMC9309660
- DOI: 10.1016/j.heliyon.2022.e09952
Defining a role for Interferon Epsilon in normal and complicated pregnancies
Abstract
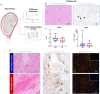
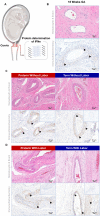
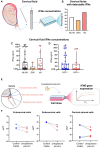
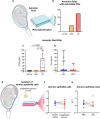
Interferon epsilon (IFNe) is a recently described cytokine that is constitutively expressed in the female reproductive tract. However, the role of this hormonally regulated cytokine during human pregnancy is poorly understood. Moreover, whether IFNe participates in host immune response against bacteria-driven intra-amniotic infection or cervical human papillomavirus infection during pregnancy is unknown. Herein, using a unique set of human samples derived from multiple study cohorts, we aimed to uncover the role of IFNe in normal and complicated pregnancies. We showed that IFNe is expressed in the myometrium, cervix, and chorioamniotic membranes, and may therefore represent a constitutive element of host defense mechanisms in these tissues during pregnancy. The expression of IFNe in the myometrium and cervix appeared greater in late gestation than in mid-pregnancy, but did not seem to be impacted by labor. Notably, concentrations of IFNe in amniotic fluid, but not cervical fluid, were increased in a subset of women undergoing spontaneous preterm labor with intra-amniotic infection, indicating that IFNe could participate in anti-microbial responses in the amniotic cavity. However, stimulation with Ureaplasma parvum and/or lipopolysaccharide did not enhance IFNE expression by amnion epithelial or cervical cells in vitro, implicating alternative sources of this cytokine during intra-amniotic or cervical infection, respectively. Collectively, our results represent the first characterization of IFNe expression by human reproductive and gestational tissues during normal pregnancy and suggest a role for this cytokine in intra-amniotic infection leading to preterm birth.
Keywords: Amniotic fluid; Cervix; Chorioamniotic membranes; IFNE; Myometrium.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hardy M.P., et al. Characterization of the type I interferon locus and identification of novel genes. Genomics. 2004;84(2):331–345. - PubMed
-
- Bourke N.M., et al. Human IFNε: spaciotemporal expression, hormone regulation and innate immunity in the female reproductive tract. bioRxiv. 2018
LinkOut - more resources
Full Text Sources

