Small Extracellular Vesicles from Inflamed Adipose Derived Stromal Cells Enhance the NF- κ B-Dependent Inflammatory/Catabolic Environment of Osteoarthritis
- PMID: 35898656
- PMCID: PMC9314187
- DOI: 10.1155/2022/9376338
Small Extracellular Vesicles from Inflamed Adipose Derived Stromal Cells Enhance the NF- κ B-Dependent Inflammatory/Catabolic Environment of Osteoarthritis
Abstract
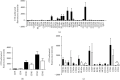
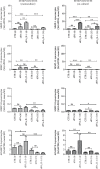
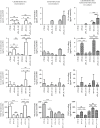
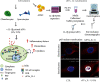
The last decade has seen exponentially growing efforts to exploit the effects of adipose derived stromal cells (ADSC) in the treatment of a wide range of chronic degenerative diseases, including osteoarthritis (OA), the most prevalent joint disorder. In the perspective of developing a cell-free advanced therapy medicinal product, a focus has been recently addressed to the ADSC secretome that lends itself to an allogeneic use and can be further dissected for the selective purification of small extracellular vesicles (sEVs). sEVs can act as "biological drug carriers" to transfer information that mirror the pathophysiology of the providing cells. This is important in the clinical perspective where many OA patients are also affected by the metabolic syndrome (MetS). ADSC from MetS OA patients are dysfunctional and "inflammatory" primed within the adipose tissue. To mimic this condition, we exposed ADSC to IL-1β, and then we investigated the effects of the isolated sEVs on chondrocytes and synoviocytes, either cultured separately or in co-culture, to tease out the effects of these "IL-1β primed sEVs" on gene and protein expression of major inflammatory and catabolic OA markers. In comparison with sEVs isolated from unstimulated ADSC, the IL-1β primed sEVs were able to propagate NF-κB activation in bystander joint cells. The effects were more prominent on synoviocytes, possibly because of a higher expression of binding molecules such as CD44. These findings call upon a careful characterization of the "inflammatory fingerprint" of ADSC to avoid the transfer of an unwanted message as well as the development of in vitro "preconditioning" strategies able to rescue the antiinflammatory/anticatabolic potential of ADSC-derived sEVs.
Copyright © 2022 Carola Cavallo et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures





References
-
- Filardo G., Tschon M., Perdisa F., et al. Micro-fragmentation is a valid alternative to cell expansion and enzymatic digestion of adipose tissue for the treatment of knee osteoarthritis: a comparative preclinical study. Knee Surgery, Sports Traumatology, Arthroscopy . 2021;30(3) - PubMed
-
- Perdisa F., Gostynska N., Roffi A., Filardo G., Marcacci M., Kon E. Adipose-derived mesenchymal stem cells for the treatment of articular cartilage: a systematic review on preclinical and clinical evidence. Stem Cells International . 2015;2015:13. doi: 10.1155/2015/597652.597652 - DOI - PMC - PubMed
-
- Cavallo C., Merli G., Borzi R. M., et al. Small extracellular vesicles from adipose derived stromal cells significantly attenuate in vitro the NF-kappaB dependent inflammatory/catabolic environment of osteoarthritis. Scientific Reports . 2021;11(1):p. 1053. doi: 10.1038/s41598-020-80032-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

