Identification of novel cyanoacrylate monomers for use in nanoparticle drug delivery systems prepared by miniemulsion polymerisation - A multistep screening approach
- PMID: 35898812
- PMCID: PMC9310130
- DOI: 10.1016/j.ijpx.2022.100124
Identification of novel cyanoacrylate monomers for use in nanoparticle drug delivery systems prepared by miniemulsion polymerisation - A multistep screening approach
Abstract
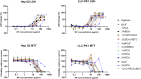
Poly (alkyl cyanoacrylate) (PACA) polymeric nanoparticles (NPs) are promising drug carriers in drug delivery. However, the selection of commercially available alkyl cyanoacrylate (ACA) monomers is limited, because most monomers were designed for use in medical and industrial glues and later repurposed for drug encapsulation. This study therefore aimed to seek out novel ACA materials for use in NP systems using a toxicity led screening approach. A multistep strategy, including cytotoxicity screening of alcohols as degradation products of PACA (44 alcohols), NPs (14 polymers), and a final in vivo study (2 polymers) gave poly (2-ethylhexyl cyanoacrylate) PEHCA as a promising novel PACA candidate. For the first time, this work presents cytotoxicity data on several novel ACAs, PEHCA in vivo toxicity data, and miniemulsion polymerisation-based encapsulation of the cabazitaxel and NR688 in novel PACA candidates. Furthermore, several of the ACA candidates were compatible with a wider selection of lipophilic active pharmaceutical ingredients (APIs) versus commercially available controls. Combined, this work demonstrates the potential benefits of expanding the array of available ACA materials in drug delivery. Novel ACAs have the potential to encapsulate a wider range of APIs in miniemulsion polymerisation processes and may also broaden PACA applicability in other fields.
Keywords: Cabazitaxel; Drug delivery; Nanoparticle characterisation; Poly(alkyl cyanoacrylate) nanoparticles; Toxicity screening.
© 2022 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Peter P. Molesworth reports financial support was provided by Research Council of Norway. Yrr Morch reports financial support was provided by Research Council of Norway. Sofie Snipstad reports financial support was provided by Research Council of Norway. Peter P. Molesworth has patent #Nanoparticles comprising copolymeric or homopolymeric compounds which comprise cyanoacrylate subunits (WO2020207655) pending to SINTEF TTO AS. Ruth Schmid has patent #Nanoparticles comprising copolymeric or homopolymeric compounds which comprise cyanoacrylate subunits (WO2020207655) pending to SINTEF TTO AS. Yrr Morch has patent #Nanoparticles comprising copolymeric or homopolymeric compounds which comprise cyanoacrylate subunits (WO2020207655) pending to SINTEF TTO AS.
Figures





References
-
- Arpicco S., et al. Recent studies on the delivery of hydrophobic drugs in nanoparticulate systems. J. Drug Deliv. Sci. Technol. 2016;32(1):298–312.
-
- Bailey S.A., Zidell R.H., Perry R.W. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol. Pathol. 2004;32(4):448–466. - PubMed
-
- Bansal V.K. In: Clinical Methods: The History, Physical, and Laboratory Examinations. HK W., WD H., JW H., editors. Butterworths; Boston: 1990. Serum inorganic phosphorus. - PubMed
-
- Bayer I.S. In: Smart Nanoparticles for Biomedicine. Ciofani G., editor. Elsevier; 2018. -5 - Nanostructured cyanoacrylates: Biomedical applications; pp. 65–81.
-
- Boraschi D., et al. Nanoparticles and innate immunity: new perspectives on host defence. Semin. Immunol. 2017;34:33–51. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

