In vitro and in vivo Characterization of Host-Pathogen Interactions of the L3881 Candida albicans Clinical Isolate
- PMID: 35898912
- PMCID: PMC9309619
- DOI: 10.3389/fmicb.2022.901442
In vitro and in vivo Characterization of Host-Pathogen Interactions of the L3881 Candida albicans Clinical Isolate
Abstract
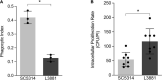
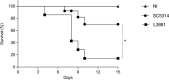
Candida albicans is a human commensal fungus and the etiologic agent of nosocomial infections in immunocompromised individuals. Candida spp. is the most studied human fungal pathogen, and the mechanisms by which this fungus can evade the immune system affecting immunosuppressed individuals have been extensively studied. Most of these studies focus on different species of Candida, and there is much to be understood in virulence variability among lineages, specifically different C. albicans clinical isolates. To better understand the main mechanisms of its virulence variability modulated in C. albicans clinical isolates, we characterized L3881 lineage, which has been previously classified as hypovirulent, and SC5314 lineage, a virulent wild-type control, by using both in vitro and in vivo assays. Our findings demonstrated that L3881 presented higher capacity to avoid macrophage phagocytosis and higher resistance to oxidative stress than the wild type. These characteristics prevented higher mortality rates for L3881 in the animal model of candidiasis. Conversely, L3881 has been able to induce an upregulation of pro-inflammatory mediators both in vitro and in vivo. These results indicated that in vitro and in vivo functional characterizations are necessary for determination of virulence in different clinical isolates due to its modulation in the host-pathogen interactions.
Keywords: Candida albicans infection; clinical isolates; fungal infection; innate immunity; virulence.
Copyright © 2022 Sucupira, Moura, Gurgel, Pereira, Padovan, Teixeira, Bahia and Soriani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Natural Variation in Clinical Isolates of Candida albicans Modulates Neutrophil Responses.mSphere. 2020 Aug 19;5(4):e00501-20. doi: 10.1128/mSphere.00501-20. mSphere. 2020. PMID: 32817378 Free PMC article.
-
A Novel Virulence Phenotype Rapidly Assesses Candida Fungal Pathogenesis in Healthy and Immunocompromised Caenorhabditis elegans Hosts.mSphere. 2019 Apr 10;4(2):e00697-18. doi: 10.1128/mSphere.00697-18. mSphere. 2019. PMID: 30971447 Free PMC article.
-
Interactions of Both Pathogenic and Nonpathogenic CUG Clade Candida Species with Macrophages Share a Conserved Transcriptional Landscape.mBio. 2021 Dec 21;12(6):e0331721. doi: 10.1128/mbio.03317-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903044 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Dissecting Candida albicans Infection from the Perspective of C. albicans Virulence and Omics Approaches on Host-Pathogen Interaction: A Review.Int J Mol Sci. 2016 Oct 18;17(10):1643. doi: 10.3390/ijms17101643. Int J Mol Sci. 2016. PMID: 27763544 Free PMC article. Review.
Cited by
-
Characterization of Oral Candida spp. Biofilms in Children and Adults Carriers from Eastern Europe and South America.Antibiotics (Basel). 2023 Apr 22;12(5):797. doi: 10.3390/antibiotics12050797. Antibiotics (Basel). 2023. PMID: 37237699 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

