Differential Gut Microbiota Compositions Related With the Severity of Major Depressive Disorder
- PMID: 35899051
- PMCID: PMC9309346
- DOI: 10.3389/fcimb.2022.907239
Differential Gut Microbiota Compositions Related With the Severity of Major Depressive Disorder
Abstract
Objective: Increasing evidence shows a close relationship between gut microbiota and major depressive disorder (MDD), but the specific mechanisms remain unknown. This study was conducted to explore differential gut microbiota compositions related to the severity of MDD.
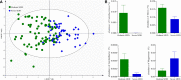
Methods: Healthy controls (HC) (n = 131) and MDD patients (n = 130) were included. MDD patients with Hamilton Depression Rating Scale (HDRS) score <25 and ≥25 were assigned into moderate (n = 72) and severe (n = 58) MDD groups, respectively. Univariate and multivariate analyses were used to analyze the gut microbiota compositions at the genus level.
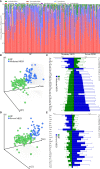
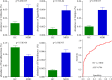
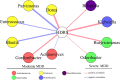
Results: Thirty-six and 27 differential genera were identified in moderate and severe MDD patients, respectively. The differential genera in moderate and severe MDD patients mainly belonged to three (Firmicutes, Actinobacteriota, and Bacteroidota) and two phyla (Firmicutes and Bacteroidota), respectively. One specific covarying network from phylum Actinobacteriota was identified in moderate MDD patients. In addition, five genera (Collinsella, Eggerthella, Alistipes, Faecalibacterium, and Flavonifractor) from the shared differential genera by two MDD groups had a fair efficacy in diagnosing MDD from HC (AUC = 0.786).
Conclusions: Our results were helpful for further exploring the role of gut microbiota in the pathogenesis of depression and developing objective diagnostic methods for MDD.
Keywords: Actinobacteriota; Bacteroidota; Firmicutes; gut microbiota; major depressive disorder.
Copyright © 2022 Zhong, Chen, Wang, Shao, Zhou and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients.J Psychiatr Res. 2019 Jun;113:90-99. doi: 10.1016/j.jpsychires.2019.03.017. Epub 2019 Mar 21. J Psychiatr Res. 2019. PMID: 30927646
-
Gut microbiota and inflammatory factor characteristics in major depressive disorder patients with anorexia.BMC Psychiatry. 2024 May 2;24(1):334. doi: 10.1186/s12888-024-05778-0. BMC Psychiatry. 2024. PMID: 38698338 Free PMC article.
-
Changes of gut microbiota reflect the severity of major depressive disorder: a cross sectional study.Transl Psychiatry. 2023 Apr 28;13(1):137. doi: 10.1038/s41398-023-02436-z. Transl Psychiatry. 2023. PMID: 37117202 Free PMC article.
-
Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder.Harv Rev Psychiatry. 2020 Jan/Feb;28(1):26-39. doi: 10.1097/HRP.0000000000000243. Harv Rev Psychiatry. 2020. PMID: 31913980 Free PMC article. Review.
-
Gut microbiota composition in depressive disorder: a systematic review, meta-analysis, and meta-regression.Transl Psychiatry. 2023 Dec 8;13(1):379. doi: 10.1038/s41398-023-02670-5. Transl Psychiatry. 2023. PMID: 38065935 Free PMC article.
Cited by
-
Gut microbiome predicts cognitive function and depressive symptoms in late life.Mol Psychiatry. 2024 Oct;29(10):3064-3075. doi: 10.1038/s41380-024-02551-3. Epub 2024 Apr 25. Mol Psychiatry. 2024. PMID: 38664490 Free PMC article.
-
The involvement of the gut microbiota in postoperative cognitive dysfunction based on integrated metagenomic and metabolomics analysis.Microbiol Spectr. 2023 Dec 12;11(6):e0310423. doi: 10.1128/spectrum.03104-23. Epub 2023 Nov 21. Microbiol Spectr. 2023. PMID: 38108273 Free PMC article.
-
Tryptophan metabolism as bridge between gut microbiota and brain in chronic social defeat stress-induced depression mice.Front Cell Infect Microbiol. 2023 Feb 24;13:1121445. doi: 10.3389/fcimb.2023.1121445. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36909723 Free PMC article.
-
Limosilactobacillus-related 3-OMDP as a potential therapeutic target for depression.Ann Med. 2024 Dec;56(1):2417179. doi: 10.1080/07853890.2024.2417179. Epub 2024 Oct 18. Ann Med. 2024. PMID: 39421970 Free PMC article.
-
The gut microbiota-brain connection: insights into major depressive disorder and bipolar disorder.Front Psychiatry. 2024 Nov 5;15:1421490. doi: 10.3389/fpsyt.2024.1421490. eCollection 2024. Front Psychiatry. 2024. PMID: 39564459 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

