Contribution of joint tissue properties to load-induced osteoarthritis
- PMID: 35899096
- PMCID: PMC9309407
- DOI: 10.1016/j.bonr.2022.101602
Contribution of joint tissue properties to load-induced osteoarthritis
Abstract
Objective: Clinical evidence suggests that abnormal mechanical forces play a major role in the initiation and progression of osteoarthritis (OA). However, few studies have examined the mechanical environment that leads to disease. Thus, using a mouse tibial loading model, we quantified the cartilage contact stresses and examined the effects of altering tissue material properties on joint stresses during loading.
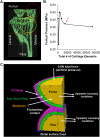
Design: Using a discrete element model (DEA) in conjunction with joint kinematics data from a murine knee joint compression model, the magnitude and distribution of contact stresses in the tibial cartilage during joint loading were quantified at levels ranging from 0 to 9 N in 1 N increments. In addition, a simplified finite element (FEA) contact model was developed to simulate the knee joint, and parametric analyses were conducted to investigate the effects of altering bone and cartilage material properties on joint stresses during compressive loading.
Results: As loading increased, the peak contact pressures were sufficient to induce fibrillations on the cartilage surfaces. The computed areas of peak contact pressures correlated with experimentally defined areas of highest cartilage damage. Only alterations in cartilage properties and geometry caused large changes in cartilage contact pressures. However, changes in both bone and cartilage material properties resulted in significant changes in stresses induced in the bone during compressive loading.
Conclusions: The level of mechanical stress induced by compressive tibial loading directly correlated with areas of biological change observed in the mouse knee joint. These results, taken together with the parametric analyses, are the first to demonstrate both experimentally and computationally that the tibial loading model is a useful preclinical platform with which to predict and study the effects of modulating bone and/or cartilage properties on attenuating OA progression. Given the direct correlation between computational modeling and experimental results, the effects of tissue-modifying treatments may be predicted prior to in vivo experimentation, allowing for novel therapeutics to be developed.
Keywords: Bone; Cartilage; Contact mechanics; Discrete element analysis; Finite element analysis.
© 2022 Published by Elsevier Inc.
Conflict of interest statement
The authors have no conflict of interest related to this work.
Figures





References
-
- Arokoski J., Kiviranta I., Jurvelin J., Tammi M., Helminen H.J. Long-distance running causes site-dependent decrease of cartilage glycosaminoglycan content in the knee joints of beagle dogs. Arthritis Rheum. 1993;36:1451–1459. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

