Oral Adverse Events Following COVID-19 Vaccination: Analysis of VAERS Reports
- PMID: 35899169
- PMCID: PMC9309565
- DOI: 10.3389/fpubh.2022.952781
Oral Adverse Events Following COVID-19 Vaccination: Analysis of VAERS Reports
Abstract
Background: Oral adverse events (AEs) following COVID-19 vaccination have been sporadically reported during the previous months, warranting further investigation for their prevalence and suspected relationship with vaccine-elicited immune response.
Methods: A retrospective analysis using the Vaccine Adverse Event Reporting System (VAERS) data was conducted to evaluate AEs within the oral cavity (mucosa, tongue, lips, palate, dentition, salivary glands) and AEs involving taste and other sensations. Oral AEs reported after receiving COVID-19 vaccination (test group) and seasonal influenza vaccination (control group) were extracted and cross-tabulated to assess their relative prevalence.
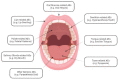
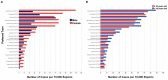
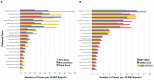
Results: Among the 128 solicited (suspected) oral AEs, oral paresthesia (0.872%) was most reported after receiving COVID-19 vaccines, followed by the swelling of lips (0.844%), ageusia (0.722%), oral hypoesthesia (0.648%), swollen tongue (0.628%), and dysgeusia (0.617%). The reported prevalence of oral AEs was higher in the COVID-19 vaccine group than in the seasonal influenza group. The distribution pattern of the most reported oral AEs was similar for both COVID-19 and seasonal influenza vaccines. Female sex, older age (>39 years old), primer doses, and mRNA-based COVID-19 vaccines exhibited a higher reported prevalence of oral AEs.
Conclusion: Within the limitations of this study, COVID-19 vaccines were found to be associated with rare oral AEs that are predominantly similar to those emerging following seasonal influenza vaccines. The most commonly reported oral AEs were oral paraesthesia (mouth-tingling), lip swelling, and ageusia, representing various pathophysiologic pathways that remain unclear. Taste-related AEs should be acknowledged in the context of the COVID-19 pandemic and the public should be adequately informed about a potential taste dysfunction after receiving the COVID-19 vaccination. Dentists and dental teams need to be aware of the prevalence, severity, and prognosis of oral AEs to inform their patients and increase public confidence in vaccines.
Keywords: COVID-19 vaccines; anaphylaxis; drug-related side effects and adverse reactions; oral manifestations; pharmacovigilance oral adverse events following COVID-19 vaccination 2.
Copyright © 2022 Riad, Põld, Kateeb and Attia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Characteristics of Oral Adverse Effects following COVID-19 Vaccination and Similarities with Oral Symptoms in COVID-19 Patients: Taste and Saliva Secretory Disorders.Med Princ Pract. 2025;34(2):101-120. doi: 10.1159/000543182. Epub 2024 Dec 19. Med Princ Pract. 2025. PMID: 39701050 Free PMC article. Review.
-
Oral adverse events following COVID-19 and influenza vaccination in Australia.Hum Vaccin Immunother. 2023 Aug;19(2):2253589. doi: 10.1080/21645515.2023.2253589. Epub 2023 Sep 21. Hum Vaccin Immunother. 2023. PMID: 37734344 Free PMC article.
-
Safety of co-administration of mRNA COVID-19 and seasonal inactivated influenza vaccines in the vaccine adverse event reporting system (VAERS) during July 1, 2021-June 30, 2022.Vaccine. 2023 Mar 10;41(11):1859-1863. doi: 10.1016/j.vaccine.2022.12.069. Epub 2023 Jan 9. Vaccine. 2023. PMID: 36669964 Free PMC article. Review.
-
Safety of Simultaneous Administration of Bivalent mRNA COVID-19 and Influenza Vaccines in the Vaccine Adverse Event Reporting System (VAERS).Drug Saf. 2024 May;47(5):487-493. doi: 10.1007/s40264-024-01406-8. Epub 2024 Feb 27. Drug Saf. 2024. PMID: 38411838 Free PMC article.
-
Oral side effects of COVID-19 vaccines in 32 European countries: Analysis of EudraVigilance reports.J Med Virol. 2023 May;95(5):e28771. doi: 10.1002/jmv.28771. J Med Virol. 2023. PMID: 37212314
Cited by
-
Characteristics of Oral Adverse Effects following COVID-19 Vaccination and Similarities with Oral Symptoms in COVID-19 Patients: Taste and Saliva Secretory Disorders.Med Princ Pract. 2025;34(2):101-120. doi: 10.1159/000543182. Epub 2024 Dec 19. Med Princ Pract. 2025. PMID: 39701050 Free PMC article. Review.
-
Otorhinolaryngologic complications after COVID-19 vaccination, vaccine adverse event reporting system (VAERS).Front Public Health. 2024 Jan 10;11:1338862. doi: 10.3389/fpubh.2023.1338862. eCollection 2023. Front Public Health. 2024. PMID: 38269374 Free PMC article.
-
Effects of post-COVID-19 vaccination in oral cavity: a systematic review.Evid Based Dent. 2024 Sep;25(3):168. doi: 10.1038/s41432-024-01014-6. Epub 2024 May 16. Evid Based Dent. 2024. PMID: 38755446
-
National Pharmacovigilance Assessment of Oral Adverse Events Following COVID-19 Vaccination in Germany (2020-2023).Int Dent J. 2025 Jul 19;75(5):100906. doi: 10.1016/j.identj.2025.100906. Online ahead of print. Int Dent J. 2025. PMID: 40684682 Free PMC article.
-
Pathophysiology of oral lesions subsequent to SARS-CoV-2 vaccination: A systematic review.J Oral Maxillofac Pathol. 2024 Jul-Sep;28(3):443-454. doi: 10.4103/jomfp.jomfp_511_23. Epub 2024 Oct 15. J Oral Maxillofac Pathol. 2024. PMID: 39670118 Free PMC article. Review.
References
-
- CDC . CDC Wonder. Vaccine Advers Event Report System. CDC (2022). Available online at: https://wonder.cdc.gov/controller/datarequest/D8 (accessed May 25, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

