Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A Prospective, Single-Center, Parallel-Group, Evaluator Blinded, Randomized Trial
- PMID: 35899211
- PMCID: PMC9309533
- DOI: 10.3389/fmed.2022.905140
Efficacy and Safety of 5% Minoxidil Alone, Minoxidil Plus Oral Spironolactone, and Minoxidil Plus Microneedling on Female Pattern Hair Loss: A Prospective, Single-Center, Parallel-Group, Evaluator Blinded, Randomized Trial
Abstract
Background: The efficacy of topical minoxidil (MX) alone on female pattern hair loss (FPHL) is limited. Combination therapy based on topical MX is currently expected to provide better outcomes.
Objectives: This study aimed to assess whether the combined therapies including MX plus oral spironolactone (SPT) and MX plus microneedling (MN) have advantages in efficacy and safety over topical MX alone on mild-to-moderate FPHL with normal hormone levels in the blood and regular menstrual cycle.
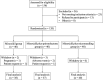
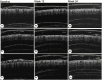
Methods: A prospective, single-center, parallel-group, evaluator blinded, randomized trial including 120 non-menopause women with proven FPHL (Sinclair class II-III) was performed in China. Patients were randomly assigned to three groups, namely, the MX group (5% topical MX alone, once daily), the MX + SPT group (MX plus SPT 80-100 mg daily), and the MX+MN group (MX plus MN every 2 weeks, 12 sessions). The change from the baseline to week 24 was assessed in hair growth (hair density and diameter under dermoscope), scalp tissue structure (epidermal thickness, dermis thickness, and average hair follicle diameter under ultrasound biomicroscopy), physician's global assessment (using a 7-point global-assessment scale and Sinclair's stage change), patient evaluation (Women's Androgenetic Alopecia Quality of Life Questionnaire and Sinclair's hair-shedding score) and side effects.
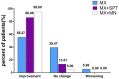
Results: In total, 115 participants completed the trial. At week 24, the hair density increased most in MX + MN group and increased least in MX group (p < 0.001 for MX + MN group vs. MX + SPT group; p = 0.009 for MX + SPT group vs. MX group). The hair shaft diameter significantly increased in all groups (p < 0.001, respectively), but there were no significant differences among the three groups (p = 0.905). The epidermal thickness and average hair follicle diameter only increased in MX + MN group. Dermis thickness increased in all groups, but there were no significant differences among the three groups. Both physician's and patient assessments showed improvement in all three groups. Scalp pruritus was the most common side effect. The MX + SPT group had the most reported adverse effects.
Limitations: The main limitations of this study are the relatively small sample size, the exclusion of severe FPHL patients, and the potential bias from unblinded treatments among the 3 groups.
Conclusion: Topical MX combined with MN is a better choice than either MX plus oral SPT or MX alone for the treatment of mild-to-moderate FPHL patients.
Keywords: efficacy and safety; female pattern hair loss (FPHL); microneedling; minoxidil; spironolactone.
Copyright © 2022 Liang, Chang, Wu, Liu, Zhao, Wang and Zhuo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer A-hW declared a shared parent affiliation with the authors to the handling editor at the time of review.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

