Safety and Biological Activity of Rozibafusp alfa, a Bispecific Inhibitor of Inducible Costimulator Ligand and B Cell Activating Factor, in Patients With Rheumatoid Arthritis: Results of a Phase 1b, Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose Study
- PMID: 35899378
- PMCID: PMC9555197
- DOI: 10.1002/acr2.11487
Safety and Biological Activity of Rozibafusp alfa, a Bispecific Inhibitor of Inducible Costimulator Ligand and B Cell Activating Factor, in Patients With Rheumatoid Arthritis: Results of a Phase 1b, Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose Study
Abstract
Objective: To assess the safety and biological activity of rozibafusp alfa, a first-in-class bispecific antibody-peptide conjugate targeting inducible costimulator ligand (ICOSL) and B cell activating factor (BAFF), in patients with rheumatoid arthritis (RA).
Methods: This phase 1b, double-blind, placebo-controlled, multiple ascending dose study included 34 patients (18-75 years; 82.4% female) with active RA (Disease Activity Score of 28 joints-C-reactive protein [DAS28-CRP] >2.6, on stable methotrexate) randomized 3:1 to receive rozibafusp alfa (n = 26, in four ascending dose cohorts of 70, 140, 210, and 420 mg) or a placebo (n = 8) subcutaneously once every 2 weeks for 10 weeks (six total doses), with 24 weeks of follow-up. The primary end point was the incidence of treatment-emergent adverse events (TEAEs). Additional assessments included serum pharmacokinetics (PK), pharmacodynamics (PD), immunogenicity, and RA disease activity measures (DAS28-CRP, Patient Global Assessment of Disease, and Physician Global Assessment of Disease).
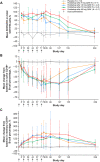
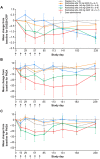
Results: TEAEs occurred in 96.2% and 87.5% of patients receiving rozibafusp alfa and the placebo, respectively; most were mild or moderate in severity. Two (7.7%) patients treated with rozibafusp alfa reported serious TEAEs; none were considered treatment related. Multiple doses of rozibafusp alfa showed nonlinear PK (mean t1/2 = 4.6-9.5 days) and dose-related, reversible PD (>90% ICOSL receptor occupancy in 210- and 420-mg cohorts; reduction in naïve B cells and increase in memory B cells in all cohorts). Five (20%) patients developed anti-rozibafusp alfa antibodies, with no apparent impact on safety. RA disease activity showed greater numerical improvement from baseline with rozibafusp alfa versus the placebo in the 210- and 420-mg cohorts.
Conclusion: Multiple ascending doses of rozibafusp alfa were well tolerated, with PK and PD reflecting dual ICOSL and BAFF blockade. Findings support further clinical evaluation of rozibafusp alfa in autoimmune disease.
© 2022 The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Catrina AI, Joshua V, Klareskog L, Malmström V. Mechanisms involved in triggering rheumatoid arthritis [review]. Immunol Rev 2016;269:162–74. - PubMed
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. - PubMed
-
- Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, et al. A RING‐type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005;435:452–58. - PubMed
-
- Her M, Kim D, Oh M, Jeong H, Choi I. Increased expression of soluble inducible costimulator ligand (ICOSL) in patients with systemic lupus erythematosus. Lupus 2009;18:501–7. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

