Optimization of trial duration to predict long-term HbA1c change with therapy: A pharmacometrics simulation-based evaluation
- PMID: 35899461
- PMCID: PMC9662199
- DOI: 10.1002/psp4.12854
Optimization of trial duration to predict long-term HbA1c change with therapy: A pharmacometrics simulation-based evaluation
Abstract
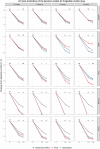
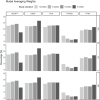
Glycated hemoglobin (HbA1c) is the main biomarker of diabetes drug development. However, because of its delayed turnover, trial duration is rarely shorter than 12 weeks, and being able to predict long-term HbA1c with precision using data from shorter studies would be beneficial. The feasibility of reducing study duration was therefore investigated in this study, assuming a model-based analysis. The aim was to investigate the predictive performance of 24- and 52-week extrapolations using data from up to 4, 6, 8 or 12 weeks, with six previously published pharmacometric models of HbA1c. Predictive performance was assessed through simulation-based dose-response predictions and model averaging (MA) with two hypothetical drugs. Results were consistent across the methods of assessment, with MA supporting the results derived from the model-based framework. The models using mean plasma glucose (MPG) or nonlinear fasting plasma glucose (FPG) effect, driving the HbA1c formation, showed good predictive performance despite a reduced study duration. The models, using the linear effect of FPG to drive the HbA1c formation, were sensitive to the limited amount of data in the shorter studies. The MA with bootstrap demonstrated strongly that a 4-week study duration is insufficient for precise predictions of all models. Our findings suggest that if data are analyzed with a pharmacometric model with MPG or FPG with a nonlinear effect to drive HbA1c formation, a study duration of 8 weeks is sufficient with maintained accuracy and precision of dose-response predictions.
© 2022 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Associate Professor Kjellsson and Mrs. Kunina received grants from Eli Lilly & Company during the conduct of the study. Dr. Chien and Dr. Garhyan are employees and stockholders of Eli Lilly & Company. All other authors declared no competing interests for this work.
Figures




References
-
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. - PubMed
-
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biomedical Information. [updated 2021 May]. Available: http://clinicaltrials.gov/. Accessed 13 Jul 2021.
-
- Cersosimo E, Triplitt C, Solis‐Herrera C, et al. Pathogenesis of type 2 diabetes mellitus. [Updated 2018 Feb 27]. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext [Internet]. MDText.com, Inc.; 2000.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

