Inhalable Formulation Based on Lipid-Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast
- PMID: 35899612
- PMCID: PMC9364311
- DOI: 10.1021/acs.biomac.2c00576
Inhalable Formulation Based on Lipid-Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast
Abstract
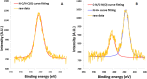
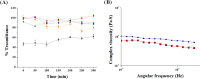
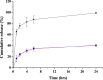
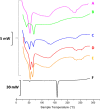
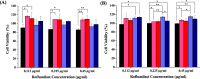
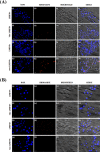
Here, novel lipid-polymer hybrid nanoparticles (LPHNPs), targeted to lung macrophages, were realized as potential carriers for Roflumilast administration in the management of chronic obstructive pulmonary disease (COPD). To achieve this, Roflumilast-loaded fluorescent polymeric nanoparticles, based on a polyaspartamide-polycaprolactone graft copolymer, and lipid vesicles, made from 1,2-dipalmitoyl-sn-glycero-3-phosphocholine and 1,2-distearoyl-sn-glycero-phosphoethanolamine-N-(polyethylene glycol)-mannose, were properly combined using a two-step method, successfully obtaining Roflumilast-loaded hybrid fluorescent nanoparticles (Man-LPHFNPs@Roflumilast). These exhibit colloidal size and a negative ζ potential, 50 wt % phospholipids, and a core-shell-type morphology; they slowly release the entrapped drug in a simulated physiological fluid. The surface analysis also demonstrated their high surface PEG density, which confers mucus-penetrating properties. Man-LPHFNPs@Roflumilast show high cytocompatibility toward human bronchial epithelium cells and macrophages and are uptaken by the latter through an active mannose-mediated targeting process. To achieve an inhalable formulation, the nano-into-micro strategy was applied, encapsulating Man-LPHFNPs@Roflumilast in poly(vinyl alcohol)/leucine-based microparticles by spray-drying.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review.Eur J Pharm Biopharm. 2013 Nov;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002. Epub 2013 Jul 17. Eur J Pharm Biopharm. 2013. PMID: 23872180 Review.
-
Diving into RNAi Therapy: An Inhalable Formulation Based on Lipid-Polymer Hybrid Systems for Pulmonary Delivery of siRNA.Biomacromolecules. 2025 Jan 13;26(1):163-177. doi: 10.1021/acs.biomac.4c00387. Epub 2024 Dec 12. Biomacromolecules. 2025. PMID: 39665463
-
Rapamycin-based inhaled therapy for potential treatment of COPD-related inflammation: production and characterization of aerosolizable nano into micro (NiM) particles.Biomater Sci. 2024 Jan 16;12(2):387-401. doi: 10.1039/d3bm01210g. Biomater Sci. 2024. PMID: 37997957
-
Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery.Int J Nanomedicine. 2015 Mar 16;10:2101-14. doi: 10.2147/IJN.S77667. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25844039 Free PMC article.
-
The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease.Pulm Pharmacol Ther. 2010 Aug;23(4):235-56. doi: 10.1016/j.pupt.2010.03.011. Epub 2010 Apr 7. Pulm Pharmacol Ther. 2010. PMID: 20381629 Review.
Cited by
-
Biomimetic Nano-Drug Delivery System: An Emerging Platform for Promoting Tumor Treatment.Int J Nanomedicine. 2024 Jan 18;19:571-608. doi: 10.2147/IJN.S442877. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38260239 Free PMC article. Review.
-
Recent Updates in Inhalable Drug Delivery System against Various Pulmonary Diseases: Challenges and Future Perspectives.Curr Drug Deliv. 2024;21(10):1320-1345. doi: 10.2174/0115672018265571231011093546. Curr Drug Deliv. 2024. PMID: 37870055 Review.
-
Lipid polymer hybrid nanoparticles against lung cancer and their application as inhalable formulation.Nanomedicine (Lond). 2024;19(25):2113-2133. doi: 10.1080/17435889.2024.2387530. Epub 2024 Aug 15. Nanomedicine (Lond). 2024. PMID: 39143915 Free PMC article. Review.
-
Nanocarriers and macrophage interaction: from a potential hurdle to an alternative therapeutic strategy.Beilstein J Nanotechnol. 2025 Jan 31;16:97-118. doi: 10.3762/bjnano.16.10. eCollection 2025. Beilstein J Nanotechnol. 2025. PMID: 39902342 Free PMC article. Review.
-
Comprehensive Aerodynamic and Physicochemical Stability Evaluations of Nanocrystal-Based Dry Powder Inhalers: The Role of Mannitol and Leucine in Enhancing Performance.Pharmaceutics. 2025 Mar 28;17(4):436. doi: 10.3390/pharmaceutics17040436. Pharmaceutics. 2025. PMID: 40284431 Free PMC article.
References
-
- Lea S.; Metryka A.; Li J.; Higham A.; Bridgewood C.; Villetti G.; Civelli M.; Facchinetti F.; Singh D. The Modulatory Effects of the PDE4 Inhibitors CHF6001 and Roflumilast in Alveolar Macrophages and Lung Tissue from COPD Patients. Cytokine 2019, 123, 15473910.1016/j.cyto.2019.154739. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

