Increased PHOSPHO1 expression mediates cortical bone mineral density in renal osteodystrophy
- PMID: 35900032
- PMCID: PMC9422252
- DOI: 10.1530/JOE-22-0097
Increased PHOSPHO1 expression mediates cortical bone mineral density in renal osteodystrophy
Abstract
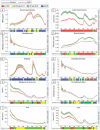
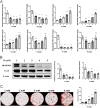
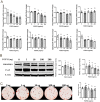
Patients with advanced chronic kidney disease (CKD) often present with skeletal abnormalities, a condition known as renal osteodystrophy (ROD). While tissue non-specific alkaline phosphatase (TNAP) and PHOSPHO1 are critical for bone mineralization, their role in the etiology of ROD is unclear. To address this, ROD was induced in both WT and Phospho1 knockout (P1KO) mice through dietary adenine supplementation. The mice presented with hyperphosphatemia, hyperparathyroidism, and elevated levels of FGF23 and bone turnover markers. In particular, we noted that in CKD mice, bone mineral density (BMD) was increased in cortical bone (P < 0.05) but decreased in trabecular bone (P < 0.05). These changes were accompanied by decreased TNAP (P < 0.01) and increased PHOSPHO1 (P < 0.001) expression in WT CKD bones. In P1KO CKD mice, the cortical BMD phenotype was rescued, suggesting that the increased cortical BMD of CKD mice was driven by increased PHOSPHO1 expression. Other structural parameters were also improved in P1KO CKD mice. We further investigated the driver of the mineralization defects, by studying the effects of FGF23, PTH, and phosphate administration on PHOSPHO1 and TNAP expression by primary murine osteoblasts. We found both PHOSPHO1 and TNAP expressions to be downregulated in response to phosphate and PTH. The in vitro data suggest that the TNAP reduction in CKD-MBD is driven by the hyperphosphatemia and/or hyperparathyroidism noted in these mice, while the higher PHOSPHO1 expression may be a compensatory mechanism. Increased PHOSPHO1 expression in ROD may contribute to the disordered skeletal mineralization characteristic of this progressive disorder.
Keywords: PHOSPHO1; TNAP; bone mineral density; bone mineralization; chronic kidney disease-mineral bone disorder; renal osteodystrophy.
Figures




References
-
- Beck-Cormier S, Lelliott CJ, Logan JG, Lafont DT, Merametdjian L, Leitch VD, Butterfield NC, Protheroe HJ, Croucher PI, Baldock PAet al.2019Slc20a2, encoding the phosphate transporter PiT2, is an important genetic determinant of bone quality and strength. Journal of Bone and Mineral Research 341101–1114. (10.1002/jbmr.3691) - DOI - PMC - PubMed
-
- Bervoets ARJ, Spasovski GB, Behets GJ, Dams G, Polenakovic MH, Zafirovska K, Van Hoof VO, De Broe ME, D'Haese PC.2003Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. American Journal of Kidney Diseases 41997–1007. (10.1016/s0272-6386(0300197-5) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

