Bacillus coagulans in Combination with Chitooligosaccharides Regulates Gut Microbiota and Ameliorates the DSS-Induced Colitis in Mice
- PMID: 35900082
- PMCID: PMC9430726
- DOI: 10.1128/spectrum.00641-22
Bacillus coagulans in Combination with Chitooligosaccharides Regulates Gut Microbiota and Ameliorates the DSS-Induced Colitis in Mice
Abstract
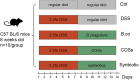
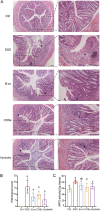
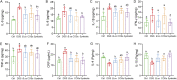
Ulcerative colitis (UC) are chronic inflammatory disorders, which may be caused by intestinal barrier dysfunction, immune system disorders and intestinal microbiota dysbiosis. Synbiotic, the combination of probiotics and prebiotics, is thought to be a pragmatic approach in mitigating inflammation in UC. Bacillus coagulans has been recognized as a potential probiotic for treating intestinal diseases because of its favorable industrial and probiotic properties, including sporulation and lactic acid production. In this study, we evaluated the treatment effects of the B. coagulans FCYS01 spores with or without the chitooligosaccharides (COSs) on UC generated using dextran sulfate sodium (DSS) in mice. Supplementation of B. coagulans spores, prebiotic COSs or the synbiotic (the spores + COSs) had a significant positive effect on DSS-induced UC. The disease activity index and histological damage score were significantly reduced after these supplementations. Compared to DSS group, these supplementations also significantly modulated the cytokines IL-4, IL-6, IL-8, IL-10, and C-reactive protein (CRP) levels and significantly maintained expressions of tight junction proteins and mucin protein and promotes recovery of the intestinal barrier. In addition, these supplementations regulate the composition of gut microbiota and improve the production of short-chain fatty acids (SCFAs), through enrichment of SCFA-producing bacteria, such as Akkermansia and Ruminococcus species. In summary, the synbiotic ameliorated the overall inflammatory status of the experimental UC model and showed a better treatment effect than B. coagulans or COSs did alone as revealed by the markers such as, colon length, IL-4 and Occludin levels. IMPORTANCE Probiotic and prebiotic are believed to be useful in alleviating the inflammatory, thereby resolving or preventing the severity of UC. Spore-forming bacteria Bacillus coagulans show advantages of stability and probiotic effects, being suggested as the important probiotics for UC treatment. Here, we demonstrate that administration of B. coagulans spores, chitooligosaccharides (COSs), or the synbiotic attenuates DSS-induced colitis and significantly correlates with altered gut immune responses. The treatment effect of the synbiotic is inferred to be relied on the enrichment of probiotic bacteria, such as Akkermansia and Ruminococcaceae species, which are reported to be crucial important for gut health. Our findings facilitate the development of therapeutic and preventive strategies for UC using spore-forming lactic acid bacteria in combination with COSs.
Keywords: Bacillus coagulans; chitooligosaccharides; microbiota; short-chain fatty acids; synbiotic.
Conflict of interest statement
The authors declare a conflict of interest. This work was supported by the National Natural Science Foundation of China (32100054 to Z.L.), the Talents Research Startup Project of Chengdu University (2081921046 to Z.L.), the Open Fund of the State Key Laboratory of Agricultural Microbiology (AMLKF202203 to Z.L.) and the Foundation of Hubei Hongshan Laboratory (2021hszd022 to N.P.). Liu Z, Jiang Z, Peng N, Zhang Z, 6 December 2021, Chinese patent, application 202111478204.2.
Figures



Similar articles
-
Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases.Eur J Nutr. 2020 Dec;59(8):3669-3689. doi: 10.1007/s00394-020-02200-9. Epub 2020 Feb 17. Eur J Nutr. 2020. PMID: 32067099 Free PMC article.
-
Synbiotic Supplementation Containing Whole Plant Sugar Cane Fibre and Probiotic Spores Potentiates Protective Synergistic Effects in Mouse Model of IBD.Nutrients. 2019 Apr 11;11(4):818. doi: 10.3390/nu11040818. Nutrients. 2019. PMID: 30979002 Free PMC article.
-
Clostridium butyricum and Chitooligosaccharides in Synbiotic Combination Ameliorate Symptoms in a DSS-Induced Ulcerative Colitis Mouse Model by Modulating Gut Microbiota and Enhancing Intestinal Barrier Function.Microbiol Spectr. 2023 Mar 28;11(2):e0437022. doi: 10.1128/spectrum.04370-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36975838 Free PMC article.
-
Exopolysaccharide of Levilactobacillus brevis M-10 Improved Physiological and Biochemical Indicators and Gut Microbiota in DSS-Induced Colitis Mice.Curr Microbiol. 2025 Mar 24;82(5):204. doi: 10.1007/s00284-025-04190-5. Curr Microbiol. 2025. PMID: 40126646 Review.
-
Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis.J Nutr Biochem. 2024 Feb;124:109505. doi: 10.1016/j.jnutbio.2023.109505. Epub 2023 Oct 26. J Nutr Biochem. 2024. PMID: 37890709 Review.
Cited by
-
Temporal dynamics and composition of ocular surface microbiota in C57BL/6J mice: uncovering a 12h ultradian rhythm.Front Cell Infect Microbiol. 2023 Nov 2;13:1244454. doi: 10.3389/fcimb.2023.1244454. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38029247 Free PMC article.
-
Sheng-Jiang powder ameliorates NAFLD via regulating intestinal microbiota in mice.Front Microbiol. 2024 May 27;15:1387401. doi: 10.3389/fmicb.2024.1387401. eCollection 2024. Front Microbiol. 2024. PMID: 38860223 Free PMC article.
-
Nutritional Interventions with Bacillus coagulans Improved Glucose Metabolism and Hyperinsulinemia in Mice with Acute Intermittent Porphyria.Int J Mol Sci. 2023 Jul 26;24(15):11938. doi: 10.3390/ijms241511938. Int J Mol Sci. 2023. PMID: 37569315 Free PMC article.
-
Combination of probiotics enhancing butyrogenesis in colonic microbiota model of patients with ulcerative colitis.Appl Microbiol Biotechnol. 2025 May 10;109(1):117. doi: 10.1007/s00253-025-13424-2. Appl Microbiol Biotechnol. 2025. PMID: 40347262 Free PMC article.
-
Effects of Bacillus coagulans on Growth Performance, Digestive Enzyme Activity, and Intestinal Microbiota of the Juvenile Fourfinger Threadfin (Eleutheronema tetradactylum).Animals (Basel). 2025 Feb 11;15(4):515. doi: 10.3390/ani15040515. Animals (Basel). 2025. PMID: 40002997 Free PMC article.
References
-
- Vindigni SM, Zisman TL, Suskind DL, Damman CJ. 2016. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therap Adv Gastroenterol 9:606–625. doi: 10.1177/1756283X16644242. - DOI - PMC - PubMed
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. 2017. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

