A Plausible Mechanism of Uracil Photohydration Involves an Unusual Intermediate
- PMID: 35900137
- PMCID: PMC9358713
- DOI: 10.1021/acs.jpclett.2c01694
A Plausible Mechanism of Uracil Photohydration Involves an Unusual Intermediate
Abstract
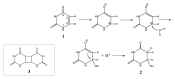
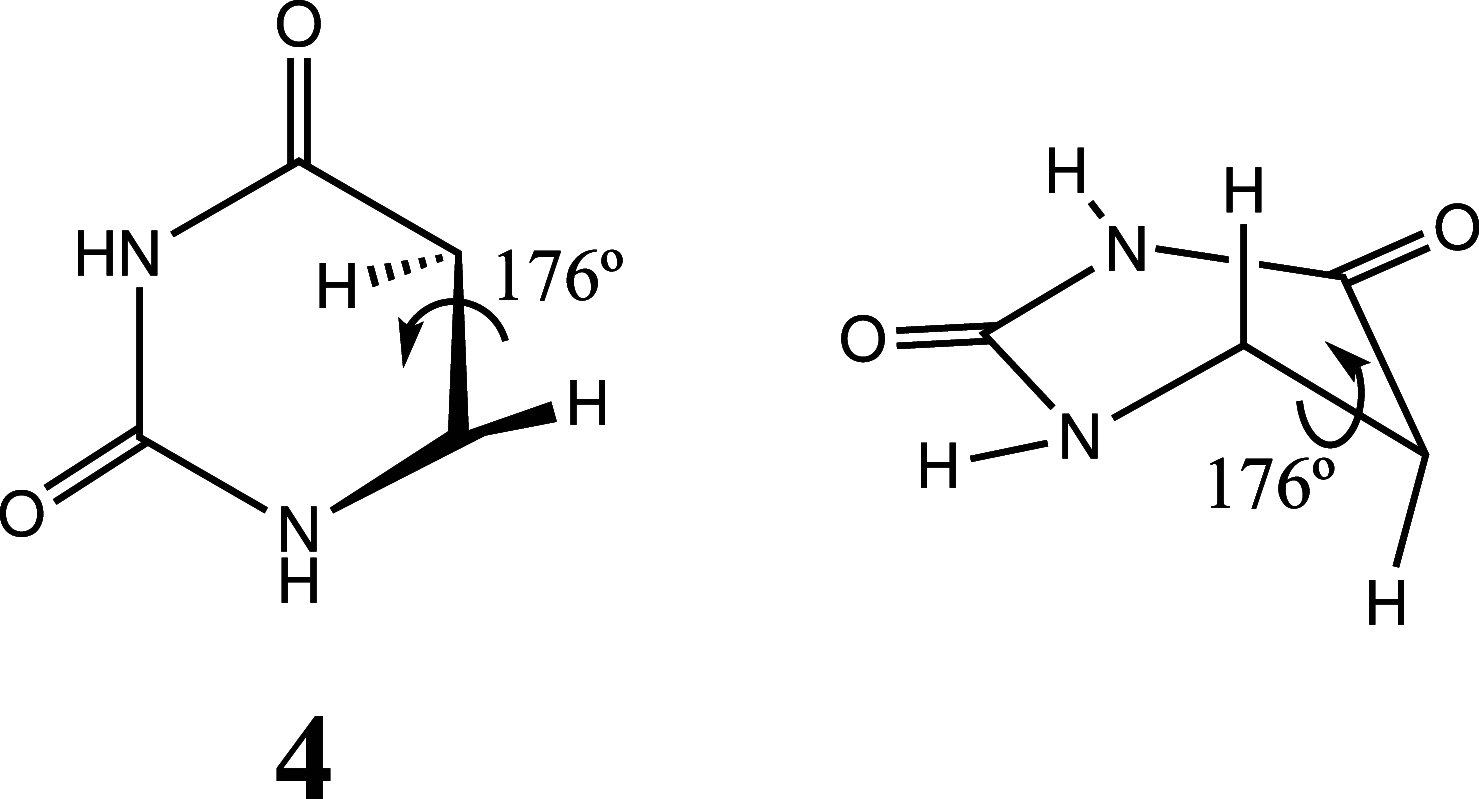
It is well-known that photolysis of pyrimidine nucleobases, such as uracil, in an aqueous environment results in the formation of hydrate as one of the main products. Although several hypotheses regarding photohydration have been proposed in the past, e.g., the zwitterionic and "hot" ground-state mechanisms, its detailed mechanism remains elusive. Here, theoretical nonadiabatic simulations of the uracil photodynamics reveal the formation of a highly energetic but kinetically stable intermediate that features a half-chair puckered pyrimidine ring and a strongly twisted intracyclic double bond. The existence and the kinetic stability of the intermediate are confirmed by a variety of computational chemistry methods. According to the simulations, the unusual intermediate is mainly formed almost immediately (∼50-200 fs) upon photoabsorption and survives long enough to engage in a hydration reaction with a neighboring water. A plausible mechanism of uracil photohydration is proposed on the basis of the modeling of nucleophilic insertion of water into the twisted double bond of the intermediate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Moore A. M. Ultraviolet irradiation of pyrimidine derivatives: II. Note on the synthesis of the product of reversible photolysis of uracil. Can. J. Chem. 1958, 36, 281–283. 10.1139/v58-038. - DOI
-
- Burr J. G.; Gordon B. R.; Park E. H. The mechanism of photohydration of uracil and N-substituted uracils. Photochem. Photobiol. 1968, 8, 73–78. 10.1111/j.1751-1097.1968.tb05847.x. - DOI
-
- Burr J. G.; Gordon B. R.; Park E. H. In Radiation Chemistry; Hart E. J., Ed.; Advances in Chemistry; American Chemical Society: Washington, DC, 1968; Vol. 81, Chapter 29, pp 418–434.
-
- Burr J. G. In Advances in the photochemistry of nucleic acid derivatives; Noyes W. A. Jr., Hammond G. S., Pitts J. N. Jr., Eds.; Advances in Photochemistry; John Wiley & Sons, Ltd., 1968; Vol. 6, Chapter 3, pp 193–299.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

