Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA)
- PMID: 35900311
- PMCID: PMC10086809
- DOI: 10.1002/jhbp.1219
Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA)
Abstract
Background: Gemcitabine/cisplatin (GC) combination therapy has been the standard palliative chemotherapy for patients with advanced biliary tract cancer (BTC). No randomized clinical trials have been able to demonstrate the survival benefit over GC during the past decade. In our previous phase II trial, adding S-1 to GC (GCS) showed promising efficacy and we aimed to determine whether GCS could improve overall survival compared with GC for patients with advanced BTC.
Methods: We performed a mulitcenter, randomized phase III trial across 39 centers. Enrolled patients were randomly allocated (1:1) to either the GCS or GC arm. The GCS regimen comprised gemcitabine (1000 mg/m2 ) and cisplatin (25 mg/m2 ) infusion on day 1 and 80 mg/m2 of S-1 on days 1-7 every 2 weeks. The primary endpoint was overall survival (OS) and the secondary endpoints were progression-free survival (PFS), response rate (RR), and adverse events (AEs). This study is registered with Clinical trial identification: NCT02182778.
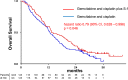
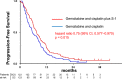
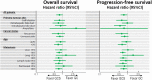
Results: Between July 2014 and February 2016, 246 patients were enrolled. The median OS and 1-year OS rate were 13.5 months and 59.4% in the GCS arm and 12.6 months and 53.7% in the GC arm, respectively (hazard ratio [HR] 0.79, 90% confidence interval [CI]: 0.628-0.996; P = .046 [stratified log-rank test]). Median PFS was 7.4 months in the GCS arm and 5.5 months in the GC arm (HR 0.75, 95% CI: 0.577-0.970; P = .015). RR was 41.5% in the GCS arm and 15.0% in the GC arm. Grade 3 or worse AEs did not show significant differences between the two arms.
Conclusions: GCS is the first regimen which demonstrated survival benefits as well as higher RR over GC in a randomized phase III trial and could be the new first-line standard chemotherapy for advanced BTC. To exploit the advantage of its high RR, GCS is now tested in the neoadjuvant setting in a randomized phase III trial for potentially resectable BTC.
Keywords: S-1; biliary tract cancer; cisplatin; gemcitabine.
© 2022 The Authors. Journal of Hepato-Biliary-Pancreatic Sciences published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Hepato-Biliary-Pancreatic Surgery.
Conflict of interest statement
TI has received personal fees from Incyte, Chugai, Yakult Honsha, Taiho Pharmaceutical, Ono Pharm, Servier, Daiichi Sankyo, Nihon Zouki, Eli‐Lilly, Otsuka, Novartis, AstraZeneca and Abbott. MK has received personal fees from Chugai. MT has received personal fees from Ono. KY has received personal fees from Ohara, Nihon‐Shinyaku, Sysmex, Chugai Pharma, Eli‐Lilly, Astra Zeneca, Otsuka, Novartis, Eisai, Nihon‐Kayaku, Boehringer Ingelheim, Taiho and Pfizer. EH has received personal fees from Eli‐Lilly and Taiho. The other authors declare that they have no competing interest.
Figures

References
-
- Lim H, Seo DW, Park DH, Lee SS, Lee SK, Kim MH, et al. Prognostic factors in patients with gallbladder cancer after surgical resection: analysis of 279 operated patients. J Clin Gastroenterol. 2013;47(5):443–8. - PubMed
-
- Marcano‐Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5(5):61. - PubMed
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

