Inhaled glucocorticoid-induced metabolome changes in asthma
- PMID: 35900313
- PMCID: PMC9346266
- DOI: 10.1530/EJE-21-0912
Inhaled glucocorticoid-induced metabolome changes in asthma
Abstract
Objective: The aim of this study was toidentify dose-related systemic effects of inhaled glucocorticoids (GCs) on the global metabolome.
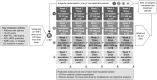
Design and methods: Metabolomics/lipidomic analysis from plasma was obtained from 54 subjects receiving weekly escalating doses (µg/day) of fluticasone furoate (FF; 25, 100, 200, 400 and 800), fluticasone propionate (FP; 50, 200, 500, 1000 and 2000), budesonide (BUD; 100, 400, 800, 1600 and 3200) or placebo. Samples (pre- and post-dose) were analysed using ultrahigh-performance liquid chromatography-tandem mass spectroscopy and liquid chromatography-mass spectrometry. Ions were matched to library standards for identification and quantification. Statistical analysis involved repeated measures ANOVA, cross-over model, random forest and principal component analysis using log-transformed data.
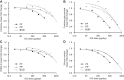
Results: Quantifiable metabolites (1971) had few significant changes (% increases/decreases; P < 0.05) vs placebo: FF 1.34 (0.42/0.92), FP 1.95 (0.41/1.54) and BUD 2.05 (0.60/1.45). Therapeutic doses had fewer changes: FF 0.96 (0.36/0.61), FP 1.66 (0.44/1.22) and BUD 1.45 (0.56/0.90). At highest/supratherapeutic doses, changes were qualitatively similar: reduced adrenal steroids, particularly glucuronide metabolites of cortisol and cortisone and pregnenolone metabolite DHEA-S; increased amino acids and glycolytic intermediates; decreased fatty acid β-oxidation and branched-chain amino acids. Notable qualitative differences were lowered dopamine metabolites (BUD) and secondary bile acid profiles (BUD/FF), suggesting CNS and gut microbiome effects.
Conclusions: Dose-dependent metabolomic changes occurred with inhaled GCs but were seen predominately at highest/supratherapeutic doses, supporting the safety of low and mid therapeutic doses. At comparable therapeutic doses (FF 100, FP 500 and BUD 800 µg/day), FF had the least effect on the most sensitive markers (adrenal steroids) vs BUD and FP.
Figures


References
-
- Global Initiative for Asthma. 2020 GINA Report, global strategy for asthma management and prevention, 2020. Available at https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-...; accessed 14 Jul 2022.
-
- Woods CP, Argese N, Chapman M, Boot C, Webster R, Dabhi V, Grossman AB, Toogood AA, Arlt W, Stewart PMet al.Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. European Journal of Endocrinology 2015173633–642. (10.1530/EJE-15-0608) - DOI - PMC - PubMed

