Resuscitation with whole blood or blood components improves survival and lessens the pathophysiological burden of trauma and haemorrhagic shock in a pre-clinical porcine model
- PMID: 35900383
- PMCID: PMC9925484
- DOI: 10.1007/s00068-022-02050-6
Resuscitation with whole blood or blood components improves survival and lessens the pathophysiological burden of trauma and haemorrhagic shock in a pre-clinical porcine model
Abstract
Purpose: In military trauma, disaster medicine, and casualties injured in remote locations, times to advanced medical and surgical treatment are often prolonged, potentially reducing survival and increasing morbidity. Since resuscitation with blood/blood components improves survival over short pre-surgical times, this study aimed to evaluate the quality of resuscitation afforded by blood/blood products or crystalloid resuscitation over extended 'pre-hospital' timelines in a porcine model of militarily relevant traumatic haemorrhagic shock.
Methods: This study underwent local ethical review and was done under the authority of Animals (Scientific Procedures) Act 1986. Forty-five terminally anaesthetised pigs received a soft tissue injury to the right thigh, haemorrhage (30% blood volume and a Grade IV liver injury) and fluid resuscitation initiated 30 min later [Group 1 (no fluid); 2 (0.9% saline); 3 (1:1 packed red blood cells:plasma); 4 (fresh whole blood); or 5 (plasma)]. Fluid (3 ml/kg bolus) was administered during the resuscitation period (maximum duration 450 min) when the systolic blood pressure fell below 80 mmHg. Surviving animals were culled with an overdose of anaesthetic.
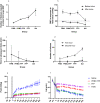
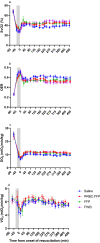
Results: Survival time was significantly shorter for Group 1 compared to the other groups (P < 0.05). Despite the same triggers for resuscitation when compared to blood/blood components, saline was associated with a shorter survival time (P = 0.145), greater pathophysiological burden and significantly greater resuscitation fluid volume (P < 0.0001).
Conclusion: When times to advanced medical care are prolonged, resuscitation with blood/blood components is recommended over saline due to the superior quality and stability of resuscitation achieved, which are likely to lead to improved patient outcomes.
Keywords: Haemorrhagic shock; Porcine model; Prolonged care; Resuscitation; Trauma.
© 2022. Crown.
Conflict of interest statement
The authors have no conflict of interest, and have no other disclosures to declare.
Figures




References
-
- North Atlantic Treaty Organisation. NATO standard AJP-4.10: allied joint doctrine for medical support edition C version 1 with UK elements. Ministry of Defence. 2019.
-
- Scallan NJ, Keene DD, Breeze J, Hodgetts TJ, Mahoney PF. Extending existing recommended military casualty evacuation timelines will likely increase morbidity and mortality: a UK consensus statement. BMJ Mil Health. 2020;166(5):287–293. - PubMed
-
- Brill JB, Tang B, Hatton G, Mueck KM, McCoy CC, Kao LS, et al. Impact of incorporating whole blood into hemorrhagic shock resuscitation: analysis of 1,377 consecutive trauma patients receiving emergency-release uncrossmatched blood products. J Am Coll Surg. 2022;234(4):408–418. - PubMed
-
- Guyette FX, Sperry JL, Peitzman AB, Billiar TR, Daley BJ, Miller RS, et al. Prehospital blood product and crystalloid resuscitation in the severely injured patient: a secondary analysis of the prehospital air medical plasma trial. Ann Surg. 2021;273(2):358–364. - PubMed
-
- Crombie N, Doughty HA, Bishop JRB, Desai A, Dixon EF, Hancox JM, et al. Resuscitation with blood products in patients with trauma-related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Haematol. 2022;9(4):e250–e261. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

